Pharma 4.0: The Ultimate Shift Toward Smarter, Safer, Faster Drug Manufacturing

Table of Content
The Pharma Industry is Changing. Fast.
AI is in the lab. Robots are on the floor. Data is the new drug.
Welcome to Pharma 4.0 – a smarter, faster, more connected era of pharmaceutical manufacturing.
This isn’t just another buzzword. Pharma 4.0 is the real-world application of Industry 4.0 principles – customized for GxP environments. And it’s changing how we develop, validate, and deliver medicines.
Pharma 4.0 envisions a fully connected, intelligent plant floor: AI-driven processes, IoT sensors everywhere, real-time data and automation, all under strict quality controls.
The benefits are compelling. Advanced manufacturing can dramatically boost efficiency and agility while ensuring product quality.
The global Pharma 4.0 market is booming. Valued at $13.7 billion in 2024 and projected to reach $40.3 billion by 2030 (CAGR ~20%).
Demand for personalized medicines and complex biologics is driving flexible, automated plants enabled by Pharma 4.0.
Compliance requirements (e.g. serialization, data traceability) force companies to adopt blockchain and IoT for supply chain transparency.
Pharma 4 represents a paradigm shift in the pharmaceutical industry, leveraging advanced technologies such as artificial intelligence (AI), machine learning (ML), Internet of Things (IoT), and blockchain to create a connected, intelligent, and automated ecosystem.
Reference here.
What You’ll Learn:
- What Pharma 4.0 actually means
- Why it matters (now more than ever)
- How top pharma companies are using it
- How you can apply it in your facility
Let’s get to work.
What is Pharma 4.0?
Pharma 4.0 is the pharmaceutical industry’s response to Industry 4.0.
It’s the smart integration of:
- Artificial Intelligence (AI)
- Machine Learning (ML)
- Internet of Things (IoT)
- Advanced Analytics
- Cloud-based systems
- Digital Twins
All connected. All validated. All compliant.
If you’re in pharma QA, manufacturing, or process validation or even cleaning validation – Pharma 4.0 is going to be the new normal.
The Revolution: From Steam to Smart Systems
Here’s the quick breakdown:
- Industry 1.0: Steam power and mechanization
- Industry 2.0: Electricity and mass production
- Industry 3.0: Computers and automation
- Industry 4.0: Smart factories, connected systems, and data-driven decision-making
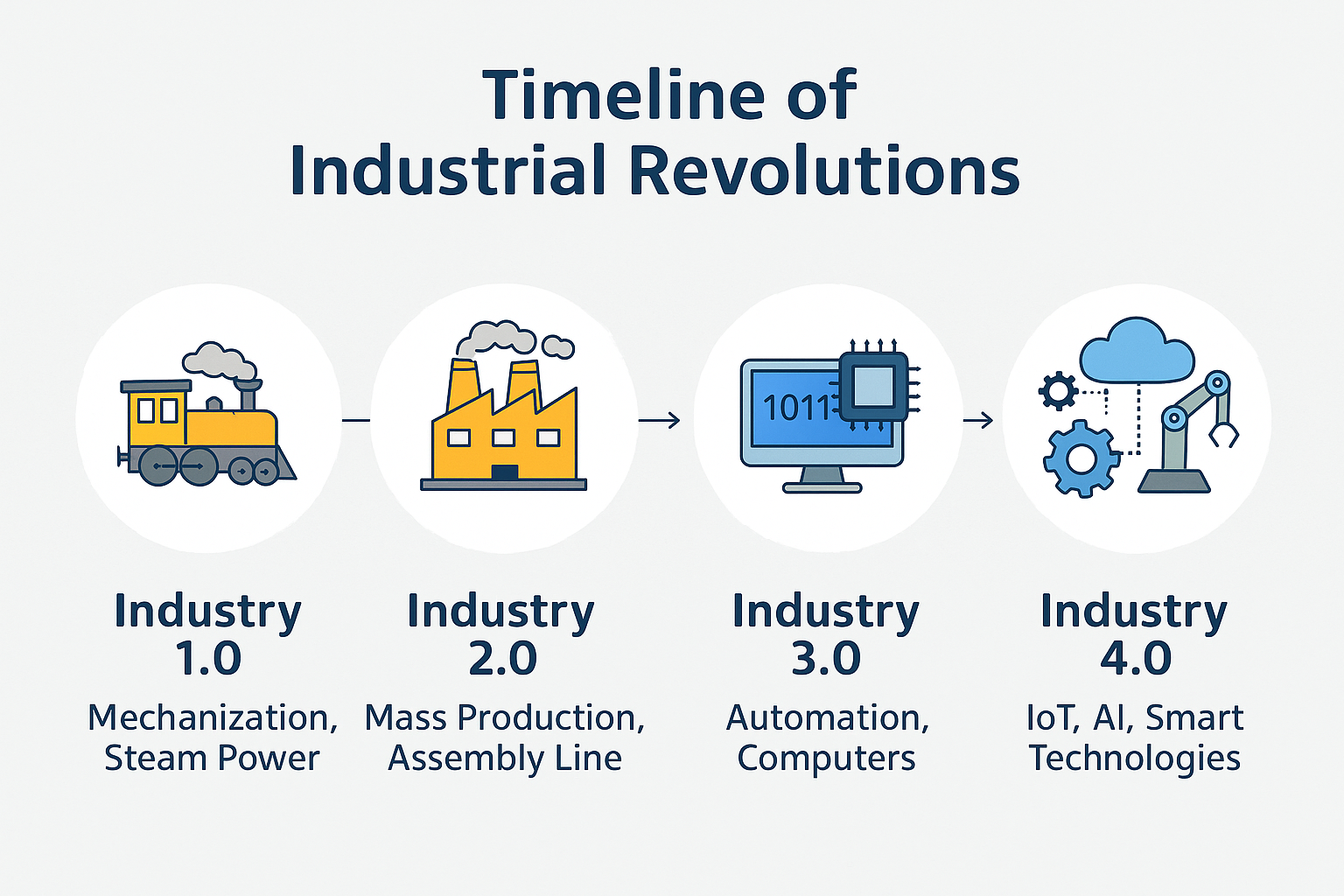
Pharma 4.0 is Industry 4.0 with compliance built in.
Even as companies embrace digital tools, strict GMP compliance and data integrity remain paramount.
Any Pharma 4.0 initiative must work within quality-by-design frameworks and regulatory guidelines (e.g., process validation, 21 CFR Part 11) – not as an afterthought but built in from the start.
It’s about maintaining GxP while getting faster, safer, and more efficient.
Key Components of Pharma 4.0 (That You Should Actually Care About)
1. Artificial Intelligence + Machine Learning (AIML)
AI is a game-changer across drug R&D and manufacturing.
In production, AI/ML can analyze millions of data points for predictive maintenance, preventing costly downtime. It can spot anomalies in real time.
Example: Pfizer’s generative AI platform now detects anomalies, boosting yield by ~10% and cutting cycle times ~25%.
On the R&D side, AI speeds drug discovery and quality control (e.g. ML for automated inspection).
Example: AI-optimized workflows at Moderna and Novartis have shown 20-30% throughput gains.
2. Big Data, Cloud & Analytics
Pharma 4.0 plants generate terabytes of data (production metrics, quality data, supply chain).
Cloud platforms and big-data analytics are used to store and analyze this flood of information.
Real-time dashboards highlight deviations; machine learning models predict failures; BI tools optimize supply flows.
Examples:
- Pfizer leverages real-time analytics in its vaccine manufacturing process to catch deviations early.
- Pfizer used AWS cloud and ML to analyze its COVID-19 mRNA production data, increasing output by 7.5% per batch.
The trend is toward digital manufacturing execution systems (MES) that unify ERP, LIMS, and automation.
3. Internet of Things (IoT)
Why it matters: IoT-enabled sensors track environmental data in real-time – temperature, pressure, humidity – feeding into QMS automatically.
IoT enables continuous monitoring: instant alerts when a mixer overheats or a temperature goes out of range.
By aggregating this data, factories attain real-time visibility (the hallmark of Pharma 4.0).
In practice, nearly one-third of top pharma companies are already deploying IoT on the plant floor. For example, Moderna used AWS IoT and ML to connect and analyze its vaccine production and supply chain sensors, automating QC and reducing manual review time.
4. Digital Twins
No more guesswork. Build a virtual replica of your process. Simulate changes. Validate faster.
Examples:
- AstraZeneca used digital twins to reduce process development time by 40%.
- At AstraZeneca’s Wuxi site, dozens of AI-driven digital twins and process simulators were used to harmonize machine schedules, contributing to a 55% output increase and 80% fewer “non-perfect” batches.
- Pfizer’s case study is a benchmark: using GPU-based CFD simulations, Pfizer built digital twins of bioreactors to predict mixing and mass-transfer, drastically cutting the number of lab trials needed.
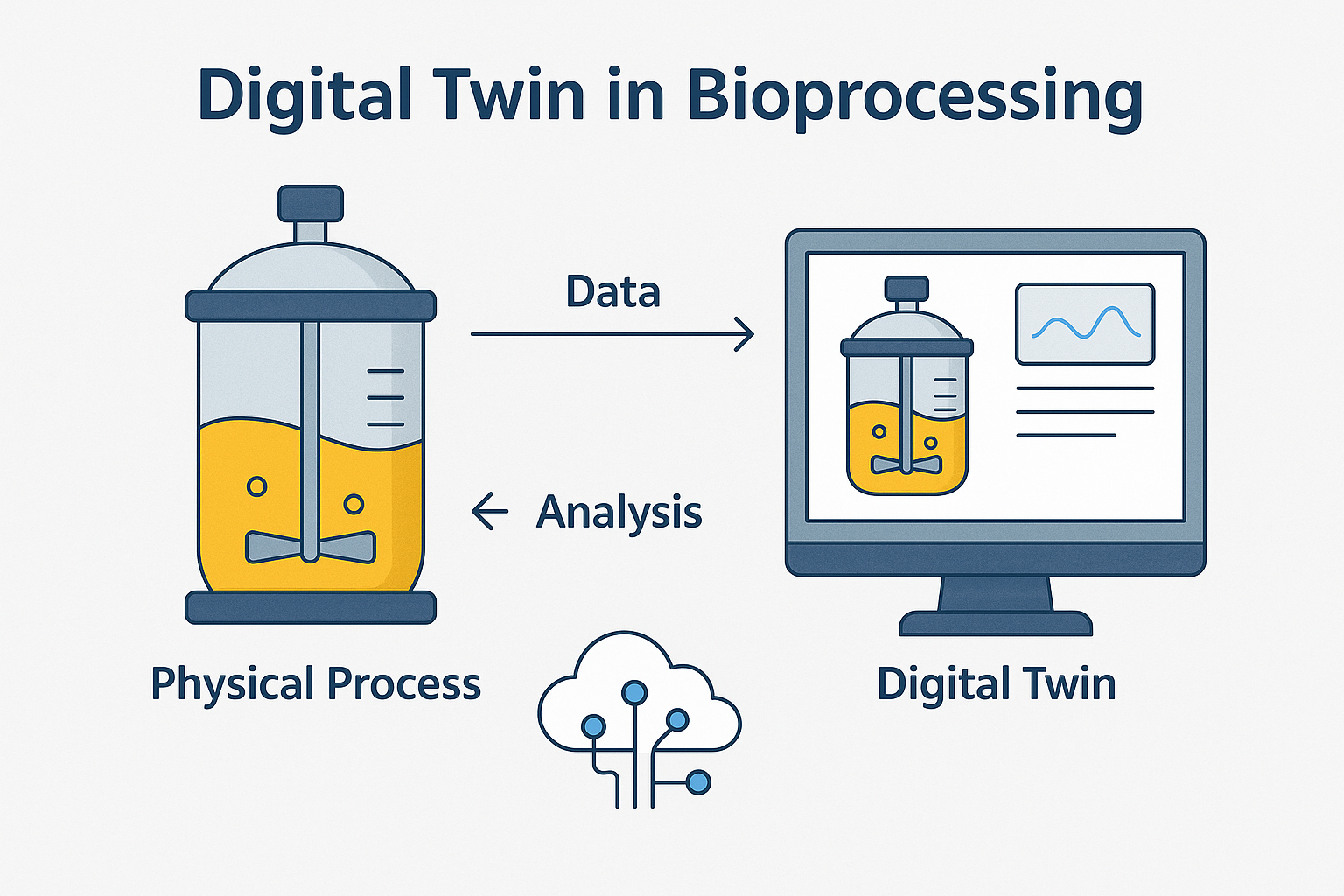
What Digital Twins Do in Pharma:
- Simulate real-world conditions without touching the physical setup.
- Predict outcomes based on process inputs and historical patterns.
- Optimize parameters (like temperature, pH, agitation) to improve yield or reduce variation.
- Support QbD (Quality by Design) and real-time release testing (RTRT).
Real Use Case:
- A biopharmaceutical company builds a digital twin of its upstream bioreactor.
- It simulates how changing nutrient flow affects cell growth and product titer.
- The team tests multiple scenarios without risking a real batch.
- It reduces batch failure, saves costs, and speeds up scale-up.
Why it matters for Pharma 4.0:
- Drives process understanding and continuous improvement.
- Enables predictive maintenance, reducing equipment downtime.
- Integrates with IoT and AI for automated insights.
5. Advanced Robotics & Automation
From line clearance to eBMR – less human error, more reproducibility.
Advanced automation – robotic arms, autonomous transport, digital work instructions – also play a role.
Examples:
- Pfizer is testing teleoperated robots (remote-controlled robots) in manufacturing to handle tasks where fixed automation or isolators aren’t suitable.
- Cobots and vision systems can do inspections, pick-and-place, or aseptic tasks with minimal human intervention. These reduce contamination risk and increase uptime.
6. Augmented Reality & Training Tech
Although less discussed, AR/VR and digital twins extend to training and maintenance. Technicians can use AR glasses to overlay equipment data or procedures in real time.
This speeds troubleshooting and ensures procedures follow electronic SOPs.
Pharma 4.0 vs Industry 4.0: What’s the Difference?
Pharma 4.0 is not a new technology trend. It’s Industry 4.0 adapted to pharma.
Unlike general manufacturing, drug production is highly regulated and risk-averse.
This means:
- Regulations Integrated: Pharma 4.0 “extends” Industry 4.0 by embedding health regulations and GMP best practices into every change. Data must meet ALCOA+ standards, and systems remain subject to FDA and EMA oversight.
- Silo-Breaking, Across Ecosystems: It breaks down silos between industry, regulators and healthcare, creating end-to-end transparency from factory floor to patient.
- Not Just IT, but Culture: Pharma 4.0 is not merely an IT upgrade. It demands organizational change: new skills, new ways of work, and human-centric design. For example, one ISPE thesis states “Pharma 4.0 is not an IT project. The operating model incorporates IT and organizational, cultural, processes & resources aspects”.
- Varying Speed: Industry 4.0 revolutionized fast-moving sectors. In contrast, Pharma 4.0 is often a slower evolution due to complexity. Companies start small – automating one line or lab – and scale up. But skipping Pharma 4.0 risks falling behind: “Pharma 4.0 is not a must, but a competitive advantage. Missing Pharma 4.0 might be a business risk”.
Here’s a quick summary:
| Feature | Industry 4.0 | Pharma 4.0 |
|---|---|---|
| Main Goal | Efficiency & Speed | Compliance + Speed |
| Sector | General Manufacturing | Life Sciences Only |
| Focus | Productivity | Product Quality & Patient Safety |
| Regulation | Low | High (GxP, FDA, EMA) |
Pharma 4.0 is not only about efficiency. It’s about TRUSTWORTHY efficiency.
The Real Power: Data Integrity by Design
If data integrity isn’t your foundation, your system is already cracking.
Data Integrity by Design is the Pharma 4.0 equivalent of Quality by Design.
It means:
- ALCOA+ is embedded into the system architecture
- Audit trails are native, not bolted on
- Every action is traceable, time-stamped, and tamper-evident
Use Cases That Actually Matter
1. Pfizer’s Smart Factory
- Real-time monitoring with IoT
- Predictive analytics cut down deviation rates by 23%
2. Merck’s Digital Twin Platform
- Used in bioreactor process optimization
- Achieved 15% increase in yield
3. Novartis’s Predictive Maintenance
- Machine learning predicts equipment failure 7 days in advance
- Saved over $4M in downtime annually
How to Build Pharma 4.0: A Step-by-Step Framework
Let’s get one thing straight: Pharma 4.0 isn’t a project. It’s a transformation.
And like any transformation, it’s not about chasing trends. It’s about building the right foundation, brick by brick.
Here’s how to actually roll it out:
Step 1: Know Where You Stand
Don’t guess. Benchmark.
Use a digital maturity model like ISPE’s Pharma 4.0 model or Acatech’s Industry 4.0 Index to get your baseline. Identify gaps in:
- People
- Process
- Technology
- Culture
Pro tip: ISPE’s Maturity Thesis #10 is gold. It helps align your organization to a clear future state.
Step 2: Set the Strategy
No tech for tech’s sake.
Link your digital plan to business goals. Think:
- Faster batch release
- Real-time quality
- Agile production
Get leadership buy-in with hard metrics. Create a 15-second pitch for your executives (yes, seriously). Make sure your QMS or PQS is audit-proof before moving forward.
Step 3: Start Smart
Don’t boil the ocean. Pick one high-impact use case.
Example:
- Predictive maintenance on key equipment
- AI vision for visual inspection
- Move one step from paper to eBMR
Treat it like a GMP project – risk assessments, validation, Part 11 compliance – all in.
Step 4: Expand with Purpose
Now scale.
Move from data capture → analytics → closed-loop control.
Use agile teams (QA + Ops + IT) and grow your digital workflows one process at a time.
Step 5: Integrate and Optimize
Once you’ve got momentum, connect your islands.
- Link MES, ERP, LIMS
- Layer in AI and digital twins
- Drive batch release decisions with predictive insights
This is where transparency turns into control.
Step 6: Build Systems That Learn
At full digital maturity, your systems:
- Self-adjust
- Flag issues in real-time
- Trigger quality alerts automatically
But you don’t need to be there to start thinking like it.
Even early wins – like ditching paper logs – can give you:
- Real-time data integrity
- Faster batch review by exception
- Fewer transcription errors
Pharma 4.0 Maturity Stages
Here’s what real progress looks like:
- Computerization: Replace paper with digital.
- Connectivity: Get your data flowing across systems.
- Visibility: Dashboards show what’s happening now.
- Transparency: Analytics reveal what’s really going on.
- Predictability: Spot problems before they happen.
- Adaptability: Systems get smarter and optimize themselves.
Each stage unlocks the next.
Validation must be part of the design. Data integrity must be built in. Every system should make your audits smoother and your outcomes safer.
Digital done right = Compliance by default.
Pros and Cons of Pharma 4.0
Pros:
Higher Efficiency & Flexibility:
Automated and optimized processes dramatically increase output. E.g., AstraZeneca boosted labor productivity by 56% .
Quality and Compliance Gains:
Real-time monitoring and analytics catch deviations early, leading to fewer defects. Electronic batch records and AI checks reduce manual error .
Data-Driven Insights:
Integrated data enables root-cause analysis, continuous improvement and informed decision-making. It enables true Quality-by-Design and real-time PQS adjustments.
Agility and Personalization:
Small-batch, personalized drug runs become feasible with flexible automation and modular (Plug-and-Produce) setups . Pharma 4.0 is a key enabler of personalized medicine.
Cost Savings:
Over time, predictive maintenance and process optimization reduce downtime and scrap. One eBMR case saw a 33% cost-per-tablet drop .
Cons:
High Upfront Investment:
Smart sensors, software, and integration have significant capital and operational costs. ROI payback can be long.
Complex Validation:
Every new digital system must be validated/qualified. Integration of IIoT devices with GxP systems increases validation scope .
Legacy Integration:
Old equipment may lack connectivity. Retrofitting or replacing is challenging.
Data Security Risks:
More connectivity means more cyber-attack surface. Pharma 4.0 programs must invest in cybersecurity.
Workforce Change:
Staff need new digital skills. There may be resistance and training burdens. Organizing cross-functional teams is non-trivial.
Overall, the business case often balances long-term benefits (safety, quality, market speed) against initial pains (cost, change). As ISPE notes: missing Pharma 4.0 might be a business risk, given competitors are already moving forward.
Frequently Asked Questions (FAQs):
What is Pharma 4.0?
Pharma 4.0 refers to the digital transformation of pharmaceutical manufacturing using AI, IoT, and automation while staying compliant with GxP regulations. In practice, Pharma 4.0 means automated batch records (eBMR), predictive analytics for processes, fully networked equipment, and digital traceability of products.
Why is Pharma 4.0 important?
It improves drug quality, reduces time to market, enhances traceability, and ensures stronger data integrity.
How does Pharma 4.0 relate to GAMP 5?
GAMP 5 (2nd Edition) guides how to validate digital systems in Pharma 4.0 environments using risk-based approaches.
Is Pharma 4.0 applicable to APIs and Biologics?
Absolutely. Biologics benefit from real-time monitoring and closed-loop control. APIs benefit from advanced analytics and predictive quality tools.
What technologies enable Pharma 4.0?
Key technologies include AI and ML (for predictive maintenance, quality inspection, process optimization); IoT sensors (real-time tracking of equipment and environment); Digital Twins (virtual models of processes for simulation and scale-up); Blockchain (secure supply chain traceability); Electronic Batch Records/MES (paperless compliance); and Cloud & Analytics (big data platforms). Robotics and AR/VR are also used for automation and training.
What is the Pharma 4.0 implementation roadmap?
1. Computerize manual steps – Digitize SOPs, e-BMRs, Validations, Process
2. Integrate these systems
3. Build Visibility & Transperancy (eg. Dashboards showing live metrics & analytics)
4. Predictability & adaptability – Rectifying the issues smartly
In practice, form a cross-functional team, start with one pilot (e.g. predictive maintenance or workflow automation), then iterate. Use agility – small steps and ‘learn-as-you-go’ to scale up while maintaining compliance.
Final Word
Pharma 4.0 is already here.
And if your systems are not yet connected, validated, and smart – you are not just behind. The business is vulnerable.
Start small. Think big. Move fast.
Need help navigating your Pharma 4.0 journey? Comment down below.
