What is Cleaning in Place or CIP Cycle? How to Automate?

The cleaning of systems at their installed place is called Cleaning in Place (CIP).
While, systems, sub-components, ancillary systems, and accessories that are unable to clean in their dedicated space or required to be dismantled and cleaned outside are called Cleaning out of Place (COP).
A successful CIP cycle makes the systems free from previous batch residues. It also improves the efficiency of production cycle time. However, to achieve this, CIP requires solid automated control.
Establishing and demonstrating an effective cleaning procedure requires detailed cleaning validation studies. We’ll just stick to the Clean In Place (CIP) system here.
Table of Content
Typical Cleaning In Place Cycle Steps
CIP is carried out after completion of a batch for equipment to remove the product-related residue (typically called soiling).
Every healthcare industry develops and employs its own cleaning procedures. Common utilities for cleaning in place are Water For Injection (WFI), Purified Water (PW), Potable Water (PoW), etc. depending on the type of product.
e.g. WFI for Biosimilars, Parenteral products, Sterile formulations, etc. PW for API formulations and PoW for Intermediates, and less stringent product domains.
Due to product complexities, we can’t define a fixed set of operations in cleaning in place cycles.
Though, the following are commonly performed CIP operations.
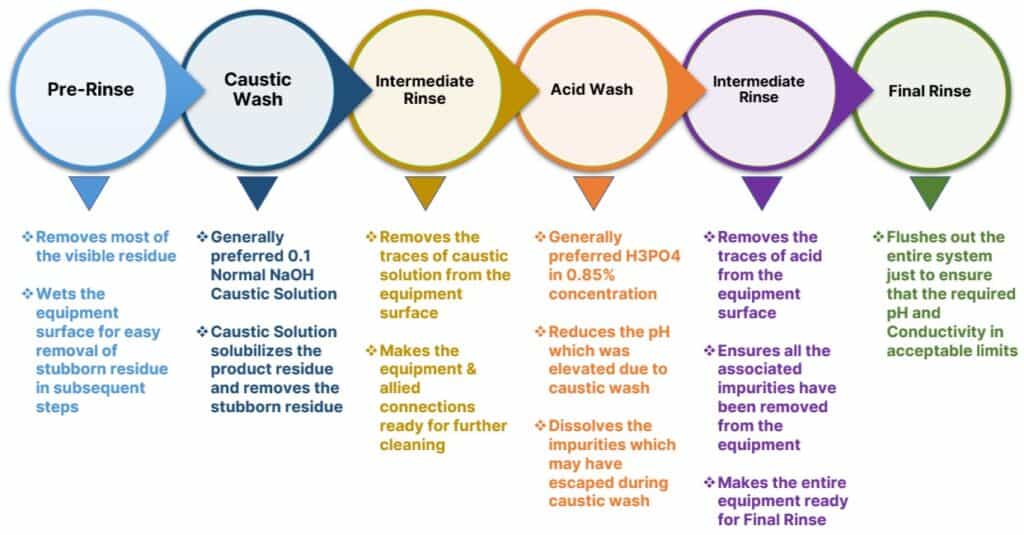
Meaningfully, a typical approach for developing a Clean-In-Place cycle is:
- Water Rinse
- Detergent Cleaning
- Post-detergent rinses.
Traditional vs. Automated
Before drug manufacturers adapted to automation, CIPs were manual. From preparing cleaning solutions to final rinsing. The measurements were also offline.
As a result, assuring the same cleaning effect every time was a challenge.
Automated CIP cycles overcame this.
Instead of discussing the difference between traditional and automated approach theoretically, see the below table.
| Traditional Approach | Automation Approach |
|---|---|
| High product contamination chances | Reduced Product Contamination |
| Longer cleaning cycle times | Short cleaning Cycle Times |
| Increased regulatory concerns | Less regulatory concerns |
| Maintaining offline measurement systems | Replaced with online measurement |
| Longer production cycle times | Shorter production cycle times |
| Consistency and repeatability is a challenge | Similar cleaning effects every time |
| Chemical exposure to operating personnel | Exposure free operations |
| The trouble with resource optimization | Resources such as WFI can be optimized effectively |
How to Design Clean-in-Place Cycles?
Designing a CIP cycle that effectively removes the product residue requires an exhaustive study of the chemical and physical dynamics of the cleaning agents and equipment surface.
In short, here are the step-by-step design aspects.
1. System Design
- Choose the system that facilitates cleaning.
- Include minimum dead legs in the system (refer ASME BPE).
- Slope the pipes towards the nearest drain points.
- Take into account, the complete coverage of the cleaning agent for product-contact surface.
- Simplify the different piping clusters into small chunks to define proper cleaning flowpaths.
2. Process Planning
- Estimate the time a product spends in the equipment and time it takes to transfer to the next system.
- Based on this, decide whether you want to go a single equipment CIP or CIP of equipment along with the transfer line. A common way is to consider equipment CIP along with the transfer line. Because it reduces the overall production turnover.
3. Piping Design
- Select the piping clusters that avoids major pressure drops by correctly estimating pipe sizes and lengths.
- Ensure the each selected piping cluster includes a low-point gravity drain.
4. Characterize the Residue
- Conduct cleaning studies or take lab-scale cleaning data to begin the residue characterization.
- Develop the parameters targetting the product-contact surface areas. Evaluate the most effective cleaning agent.
- Identify the worst-case residues, CQAs and CPPs for a cleaning process.
- Once the cleaning parameters identified, combine the CIP for similar cleaning parameters to save the time and effectively clean the system.
5. Automation-Based Design Approach
- Sequence should start with WFI/PW/PoW rinse followed by detergent cleaning and finally post-detergent rinses.
- Include draining sequence in between the rinses to systematically isolate different cleaning agents.
- Finally, Air blow step is suggested post-draining for faster gravity drains.
- Define the phases and step transition criteria in a Clean-In-Place sequence. For example,
- Exposure time
- Cleaning agent’s concentration
- Process temperature
- Mechanical actions such as agitation
- Conductivity etc.
If the above steps are followed systematically, it will significantly reduce the number of CIP trials during detailed engineering.
Automated Cycle
To easily understand the automation philosophy, let us see the basic classification of the systems.
- Mobile Systems (Movable on the floor)
- Stationary Systems (Fixed directly on the floor)
Also, there are two ways to perform cleaning in place of the equipment.
- Using CIP Skid
- Using an in-built CIP cycle
Drug manufacturing uses complex process systems and piping clusters. Every piece of equipment may undergo either dedicated or an integrated cleaning in place treatment.
Here dedicated means the CIP of an individual system. And integrated means the CIP of multiple systems through a single recipe.
To make it easy, we’ll look at the in-built CIP of a dedicated process vessel. While cleaning steps remain constant, the philosophy for an integrated approach is different than single-vessel CIP.
Just to give you a rough idea.
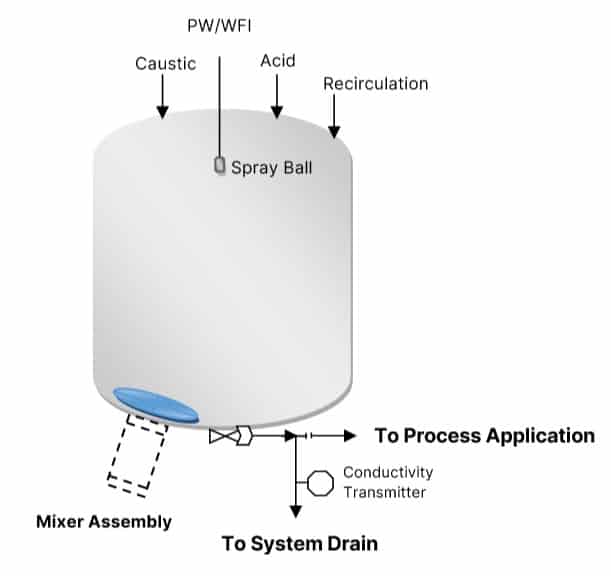
Recipe Based Approach
A recipe is a step-by-step operation that behaves as per user set points.
You manage the recipe effectively, it will manage the outcome effectively.
For example, a typical recipe for the CIP of a 600L working volume process vessel looks like this:
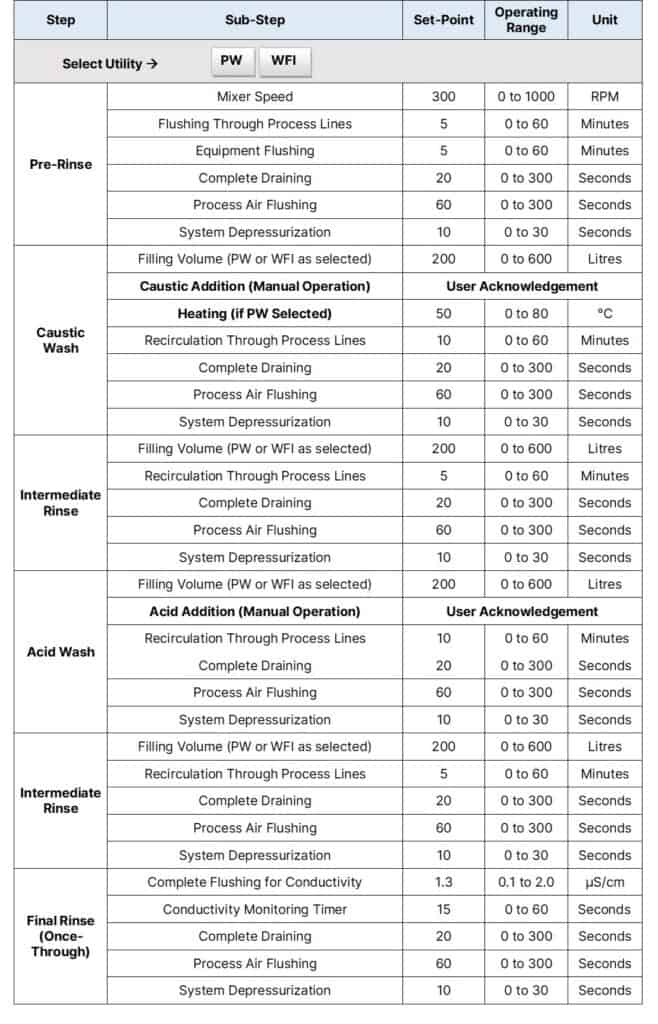
To perform CIP of equipment, separate caustic and acid solution dosing systems are required. While using CIP skids, the skid itself takes care of it. Hence, the above steps may be modified.
Before recipe loads, system-readiness checks are very important like:
- Input/Output testing
- Availability of cleaning agents
- Pressurized conditions of clean utility such as WFI
- Drain lines are properly connected
- Equipment is empty and in “To be cleaned” status
Once the recipe loads successfully, it is ready to execute.
Execution
Based on the selected utility, the cleaning process is initiated. Purified water, if selected, would require heating during the caustic wash to effectively disengage high soiling at high pH.
Whereas, WFI itself is in hot condition approx. 80°C and hence not included in a recipe. The mixer is kept ON till the completion of the cycle at a suitable RPM.
To avoid a dry run of a mixer, have min. working-level interlock in place.
Pre-Rinse
- PW or WFI introduced in the system through the main header and then sub-header. Flushing of the vessel along process lines ensures large-sized soiling or residue is easily removed.
- The soiled water then leaves the system through the drain point and therefore this step is once through. The effectiveness of this step can be confirmed by installing a suitable turbidity meter in drain locations.
- Upon complete draining, process-grade air is flushed through the system to ensure no moisture remains inside the surface of the piping and equipment.
- The system then depressurizes and gets ready for the next step.
Caustic Wash
Caustic wash is also called alkali wash.
- PW or WFI is filled to the set point.
- Caustic has been added to prepare a 0.5N NaOH solution. Explained further on how to make it 0.5N.
- If PW is used, then the system is heated to the temperature range of 60-80°C with the help of an appropriate heating medium through the jacket. In the case of WFI, this step is not required.
- Once the solution is heated, it is then recirculated through various process lines for a set time to ensure all the residue is dissolved and disengaged from product contact surfaces.
- After recirculation, the system is drained completely.
- Upon complete draining, process-grade air is flushed through the system to ensure no moisture remains inside the surface of the piping and equipment.
- The system then depressurizes and gets ready for the next step.
Intermediate Rinse
- PW or WFI is filled to the set point.
- Recirculation is done from the same path that was employed during the caustic wash to ensure remaining residue traces were removed along with the caustic solution.
- After recirculation, the system is drained completely.
- Upon complete draining, process-grade air is flushed through the system to ensure no moisture remains inside the surface of the piping and equipment.
- The system then depressurizes and gets ready for the next step.
Acid Wash
- PW or WFI is filled to the set point.
- Acid is added to prepare 0.05N Ortho-Phosphoric Acid solution. Explained further on how to make it 0.05N.
- It is then recirculated through process lines for a set time. Protein residue that may have escaped from caustic wash gets removed here.
- After recirculation, the system is drained completely.
- Upon complete draining, process-grade air is flushed through the system to ensure no moisture remains inside the surface of the piping and equipment.
- The system then depressurizes and gets ready for the next step.
Intermediate Rinse
Similar to the previous intermediate rinse.
Final Rinse (Once-Through)
- The system and process lines are flushed with PW or WFI and drained simultaneously for a set time to remove all the residue of cleaning agents accumulated during cleaning.
- pH and conductivity are monitored during flushing. Sometimes, conductivity can dictate that equipment and associated process lines are free from any residue.
- The conductivity of less than or equal to 1.3 µS/cm will terminate the cycle upon successful monitoring for the set time.
- Once the conductivity is achieved, the system is drained completely.
- Upon complete draining, process-grade air is flushed through the system to ensure no moisture remains inside the surface of the piping and equipment.
- The system then depressurizes and gets ready for the next batch.
Cleaning in Place is considered complete now. Intermediate rinses add one layer of cleaning assurance and can be considered optional.
If the critical process parameters for pharmaceutical cleaning operations are not meeting the acceptable limits, the cleaning cycle will have to be re-executed.
Following are the critical process parameters generally considered for cleaning in place.
- Flowrate
- Pressure at Utility Supply
- Temperature and sometimes pH
- Acid and Caustic concentrations
- Acid and Caustic contact times
- Final rinse Conductivity demonstrating all cleaning agents removed completely
- Sometimes TOC
In short, a successful CIP should meet the quality of the WFI at the end.
Velocity is the Key
To achieve the best cleaning results, velocity should range between 1.5 to 2 m/s.
To check this, having a flowmeter with velocity measurement at the recirculation pump supply side can be considered.
A velocity <1.5 m/s is considered a laminar flow. Hence, would not promote proper cleaning. While a velocity >2 m/s would not obtain additional significant effectiveness.
Importance of Cleaning Agents
The selection of a cleaning agent plays an important role in the effective cleaning of your healthcare equipment.
Finished product processing plants generally use 0.5N NaOH as a caustic and 0.05N OPA as the acid solution. They are adequate in dissolving proteins and other commonly associated residues.
Please note, they can’t remove scaling formed on the surface of the equipment or process lines. For that purpose, we generally carry out passivation with the help of Per-acetic acid or Nitric acid.
How to prepare 0.5N Caustic Solution?
The caustic solution is generally 0.5N. Consider the dissolution of 20g of NaOH in 1L of PW to calculate the total required quantity of NaOH. Simply multiply the cleaning volume by a factor of 20 and that will give you the total grams of required NaOH.
Grams of NaOH required = 20 * (Cleaning Volume)
You can calculate this for solid compounds pretty straight, but liquids like acids need additional calculations.
How to prepare 0.05N Ortho Phosphoric Acid?
Commonly practiced acid for CIP is 85% concentrated ortho-phosphoric acid (OPA) of 0.05N.
In the case of 0.5N Ortho Phosphoric Acid (H3PO4), a simple way to calculate the required amount of acid is to multiply by a factor of 1.137 (arrived to this value after some calculations).
So the formula is:
Volume of H3PO4 required (ml) = 1.137 * (Volume of Total Solution Including Acid (L))
The concentration and composition of cleaning agents used should PROVE the effectiveness of your cleaning in place cycle.
Find Optimization Opportunities
Once set does not mean settle. Find opportunities to optimize your existing cleaning cycles both time and cost-wise (ofcourse, without hampering the outcome).
Companies should periodically monitor their cycle behaviors and trends. Trends help in finding potential risks and opportunities to improve.
After that, the recipe parameters should come on your CIP optimization plan.
Excessive cleaning is nothing but a waste of time and resources. A retrospective study of your current CIP cycles would definitely bring something new to the table to optimize.
Riboflavin or Spray Ball Coverage Test
Purpose:
This test is used to evaluate the cleaning effectiveness of the spray ball for particular equipment. Moreover, to ensure the cleaning in blind spots of the equipment created by shadows due to agitator, baffles, nozzles, mechanical fittings, etc.
Scope:
This cleaning pattern applies to the Cleaning In Place (CIP) of process vessels, mainly demanding documented evidence of cleaning effectiveness.
Pre-requisites:
- As part of the procedure, equipment must already be cleaned to avoid false reporting of fluorescence under ultra-violet light.
- Riboflavin solution with the required quantity filled into the spray device. Prepare Riboflavin solution by dissolving 1 gm of Riboflavin in 10 Lit. water for 100ppm solution.
- CIP Pump on the supply side to deliver at least 2.5 to 3 bar(g).
- Electropolishing has been completed and approved.
- Availability of pressure gauge, flow meter, and control valves.
- Identify and define critical or worst-case areas of the equipment.
Background:
Riboflavin is most widely used for this test. It is a yellow vitamin that gives fluorescence under UV light. It is mixed with water and sprayed on the inside surface of the equipment being tested for cleanability. Rinsing is then delivered through the spray ball in a burst-rinse method.
Maintain the designed flow and philosophy of cleaning when performing the riboflavin test. The cleaning in place cycles must run under the bracketing of system design to confirm the results as repeatable.
Procedure:
Steps to perform riboflavin testing:
- Spray the solution on the inside surface of the equipment including all critical areas.
- Perform cleaning through a spray ball at the specified flow rate, time, pressure, and frequency.
- Inspect visually with the help of UV light for any fluorescence post-cleaning.
- Note down the observations and conclude as pass/fail.
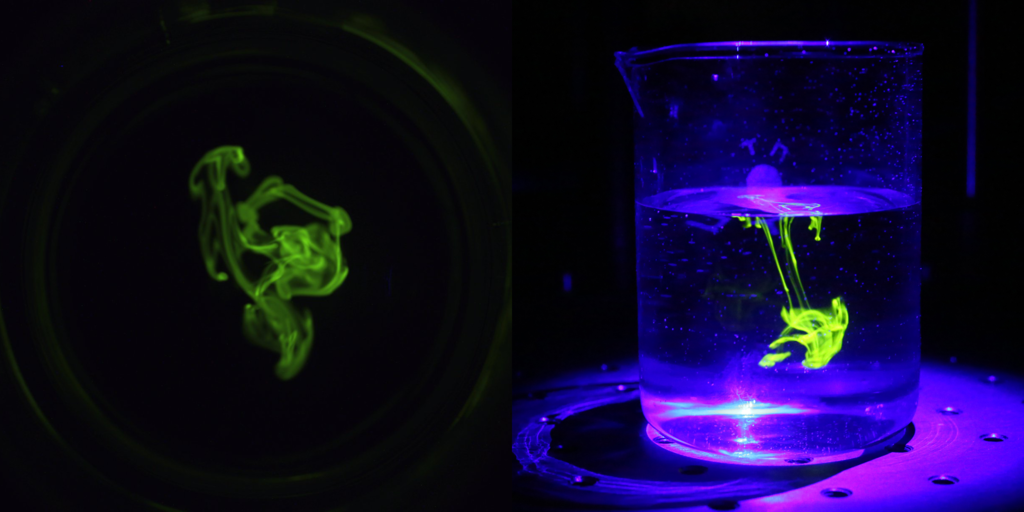
If riboflavin is still present post-cleaning, then it would appear like the below picture under UV light and the cycle would be considered a failure. Else pass.
Conclusion
Designing and implementing automated cleaning in place cycles is a complex process. To make it flawless, automation is the best way.
Though development platforms and measurement systems have been upgraded over time, the fundamentals still are the same.
The above explanation is just the tip of an iceberg and actual digging starts with design and execution case-by-case.
How does automation help you ensure the system is effectively cleaned? Or are you still doing CIP manually? Either way, comment below.

Hello Saket: Thanks for sharing the CIP article, I am working in a small OTC and cosmetic manufacturer that needs to comply with FDA cleaning and validation; as the batching is completed, vessels and filling lines are cleaned for next production batches; in 15 years there were no mayor contamination issues, with the company growth and post COVID-19 concerns, we would like to update our protocols. If possible could you refer us reading material leading to latest requirements, sort of the must have Cleaning and Cleaning Validation SOPs? Thanks in advance! FP
Thanks Francesca! Yes, I have a well-written article on the Cleaning Validation that you might want to go through. Here’s the link and let me know how it goes…
https://pharmagxp.com/quality-management/cleaning-validation/
Hello Dear,
I’m Ehsan Rad from Iran, can you share with me some documents to Cleaning Validation Methods? Because I’m working in the Quality department of the pharmaceutical industry.
Best Regards.
Hello Ehsan, thanks for connecting. I would recommend this guide for cleaning validation for you. Kindly go through and let me know if you need any further assistance.
https://pharmagxp.com/quality-management/cleaning-validation/