Fh Value Calculator Useful in Dry Heat Sterilization

Thermal sterilization isn’t just about applying heat, it’s about precision. And that’s where the Fh Value comes in. This metric helps determine the lethality of a heat treatment in Dry Heat Sterilization (DHS), ensuring consistent sterilization cycles without compromising product integrity.
There are different types of sterilization mechanisms: Steam Sterilization, Dry Heat Sterilization, Ethylene Oxide Sterilization, Depyrogenation, etc.
Choosing the right method of sterilization depends on the material being sterilized and the purpose of sterilization.
Steam sterilization is done under high temperature and high pressure in moist conditions. Hence it takes less time to sterilize.
Whereas, dry heat sterilization is done under high temperature and in dry condition and hence takes more time to sterilize or remove all forms of microorganisms.
Most of these techniques carry their own effectiveness values (explained further).
In the F0 value calculator, we established an exposure equivalency for steam sterilization (compared the ideal and actual exposure times). And thus, the F0 value is called Equivalent Exposure Time.
Here, we’ll talk about the Fh value calculator which is in analogy with the above. But in terms of dry heat, the Fh value is called Heat Penetration Factor.
In this post, you’ll learn:
- How to calculate the Fh value.
- Using Fh value calculator.
- Differences in F0, Fh and Fd values.
- Fundamental comparison of different sterilization techniques.
Though the F0, Fh, Fd values are similar in structure, their application is different.
Simply put:
| Value | Used for Purpose of |
|---|---|
| F0 | Moist Heat Sterilization/Steam Sterilization |
| Fh | Dry Heat Sterilization |
| Fd | Depyrogenation (discussed further…) |
Ideally speaking, sterilization is successful when the product contact parts are free of viable microorganisms.
Now coming to the z-value. Remember? We assumed it as 10°C for the F0 value in steam sterilization when the exact microorganism was unknown.
Similarly, in the Fh concept, the z-value is assumed as 20°C.
When it comes to moisture-sensitive items, steam sterilization is not appropriate and may damage the accessories contacting drug products.
In such cases, Dry Heat Sterilization is the more appropriate option given that the materials withstand higher temperatures than steam sterilization conditions.
Table of Content
Formula For Fh Value
The following formula is used to calculate the Fh value in dry heat sterilization.
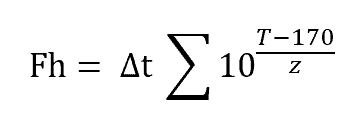
Where,
Δt – Sterilization Hold Time in Minutes
T – Average Temperature of all probes during a sterile hold in °C
z – Temperature Coefficient in °C
The term next to Δt in the above equation is lethality rate.
For steam sterilization, the term right to “T” was 121.1°C whereas, in dry heat sterilization, it is 170°C.
The Fh Value Calculator
Difference Between F0 and Fh Value
| F0 Value | Fh Value |
|---|---|
| Evaluates the effectiveness of Steam Sterilization | Evaluates the effectiveness of Dry Heat Sterilization |
| The assumed z-value is 10°C for sterilization range of 100 to 130°C | The assumed z-value is 20°C for sterilization range of 160 to 200°C |
| Theoretical Requirement 121.1°C at 30 min. of sterile hold time | Theoretical Requirement 170°C at 32 min. of sterile hold time |
| Mitigates micro-organisms, especially living endospores | Removes bacterial endotoxins |
| More complicated due to steam quality requirements | The lethality of the microbes is less than that of F0 at the same temperature |
Dry Heat Sterilization
Principle of working
Dry heat sterilization uses conduction as a mode of heat transfer. The heat is absorbed by the outside surface of an item, then passes towards its center. It will eventually reach the temperature required for sterilization.
In other words, the dry heat oxidizes the cell contents and thereby kills the organisms.
A blend of temperature and time is required to kill microorganisms by heat. In general, an increase in temperature will result in a decreased time for bacteria-kill. But, relative to steam sterilization, DHS takes more time to sterilize.
Items that can withstand high temperatures can be sterilized with Dry Heat sterilization like anhydrous (moisture-less).
There are two types of dry heat sterilization:
- Natural Convection
- Forced Convection
A quick comparison of these two.
| Natural Air DHS | Forced Air DHS |
|---|---|
| Oven-type coils transfer the heat from the bottom of the chamber by gravity convection. | Blower-driven system circulating hot air across the chamber at high velocity. |
| More of a traditional setup. | An advanced setup. |
| Requires more time to attain the sterilization set temperatures. | The rate of convection is high due to the use of a blower. Hence achieves the sterilization set temperature in a quick time. |
| Inconsistent temperature distribution inside the chamber. | Uniform temperature distribution across the chamber. |
Application of DHS:
Dry heat sterilization is particularly suitable for materials that can tolerate high temperatures and are damaged by moisture or high pressure.
Since dry heat does not cause corrosion of metal instruments and glassware, it is appropriate for sterilizing them, but it can’t be used to sterilize fabrics, plastics, or rubber.
Lastly, DHS is for materials that must remain dry. For example, glass bottles that contain pharmaceutical powders.
Depyrogenation is Different
There is a difference between Dry Heat Sterilization and Depyrogenation.
The process that removes or inactivates the pyrogens (disables their multiplication) is called depyrogenation.
In aseptic filling, depyrogenation is mainly utilized for sterilizing vials. It can also be used for sterilizing assembled and packaged materials, as heat conduction is not dependent on contact with steam or water.
In the 2nd case, the temperature is even more than DHS and the Fh value is then replaced by the Fd value.
Fd value is a function to measure the effect of the depyrogenation. If we say Fd value to be 2, the effect achieved is equivalent to the exposure of 250°C at 2 mins.
Meaning, as F0 value is for Steam Sterilization, Fd value is for Depyrogenation.
The theoretical requirement for Depyrogenation is 250°C at 30 min. with the z-value assumed as 46.4°C (see the comments). Though this is an ideal condition, a successful depyrogenation would also establish when the system achieves ≥ 3 log reduction.
Whereas, Dry Heat Sterilization occurs at 170°C for 32 min.
Simply put:
| Parameter | Dry Heat Sterilization | Depyrogenation |
|---|---|---|
| Value | Fh value | Fd value |
| Formula difference | T -(minus) 170°C | T-250°C |
| z-Value | z = 20°C | z = 46.4°C |
Conclusion
Sterilization conditions can vary based on bacterial behavior. But the fundamentals? They stay the same. Mastering Fh value calculations ensures your sterilization cycles are effective and reliable.
Whether in process design or routine operations, these values are critical – so don’t overlook them.
You can use this calculator to evaluate probable scenarios in your DHS-related operations once validated.
Now, over to you:
How do you evaluate the Fh value? Using validated software or pre-validated sheets?
Drop a comment below and let’s talk.



When Calculating Fd lethality, do we usually use a D-value of 4.99 @ 250 degree C reference? I understand that is referenced on Tsuji and Harrison studies, but is this what everyone is using during calculations or generating reports for Fd?
You’re right. For dry heat sterilization, the reference is 250°C, with Tsuji & Harrison reporting a D-value of ~4.99 min.
That number is often used as a baseline in Fh calculations, but not everyone applies it blindly. Some reports use it as a conservative literature value, while others determine organism-specific D-values through challenge studies.
The critical part is consistency and justification. If you cite 4.99, you need to reference the source and explain why it applies to your load and cycle. If you generate your own D-value, show the data. Both approaches are acceptable, as long as the rationale is documented.
Where did you obtain your values for an effective depyrogenation cycle being 30 minutes at 250C to produce a 3-log reduction? I believe that the Z value data that you reference comes from Tsuji and Harrison, and they use a D value for 1 log reduction of 4.99 minutes at 250C. This would mean that a 3-log reduction would be accomplished in 15 minutes at 250C. How did you arrive at a time of 30 minutes for the three-log reduction requirement?
Great question and you’re absolutely right to dig into the math.
The 30-minute cycle at 250°C is an industry-adopted worst-case approach, not a literal D×3 calculation. While Tsuji and Harrison provide a D-value of 4.99 minutes at 250°C, regulators often expect overkill conditions to ensure margin in real-world variability.
That’s why 30 minutes at 250°C is widely referenced, not because it’s minimal, but because it’s robust.
Appreciate your sharp observation: This nuance deserves to be understood more often.
Hi, Mr. Saket Yeotikar
Thanks for this organized article. But after reviewing the comments, I’m not understanding yet the basis for Z value 46.4°C in depyrogenation; 20°C in DHS. Could you share the basis of these preferable Z values and any calculative way to verify these values.
i am also having same question. on what basis 46.4 degree c is considered?
Great question Hossain & Syamala. You’re not alone in asking it.
Here’s the breakdown:
• Z-value of 46.4°C for depyrogenation comes from experimental work on endotoxins (specifically LPS). Studies, including Tsuji and Harrison, showed that for each 46.4°C increase, the D-value drops by 1 log. This is specific to endotoxin destruction in dry heat, which behaves differently from microbial kill.
• Z = 20°C is used for microbial inactivation (like bacterial spores), often applied in dry heat sterilization (DHS) when you’re not targeting endotoxins but sterility.
No magic in the numbers, they’re based on empirical plots of log D vs temperature. If you plot that curve, the Z-value is simply the temperature change needed to shift the D-value by a factor of 10 (log reduction).
Want to verify it? Plot log(D) on the Y-axis, temperature on the X-axis: the slope will tell you the Z.
In short:
• 46.4°C → Pyrogen reduction
• 20°C → Microbial kill
They’re not interchangeable, and that’s why mixing them creates confusion.
I have a question regarding GMP and the question says
The risks that GMP is designed to reduce are:
(A) In principle mix-ups and cross-contamination.
(B) Wrong labeling of the finished pack only and cross-contamination.
(C) Expected cross-contamination and mix-ups.
(D) Injury or death due to side effects of the drug.
which is the best answer
Let’s break it down like a GMP pro:
✅ Correct Answer: (A) In principle mix-ups and cross-contamination.
Here’s why:
GMP (Good Manufacturing Practice) is all about protecting product quality and patient safety. The two primary risks it controls are:
1. Mix-ups: Wrong material, wrong process, wrong product.
2. Cross-contamination: One product contaminating another, especially in multiproduct facilities.
Let’s eliminate the other options:
(B) is too narrow. GMP is not limited to “wrong labeling only.”
(C) mentions “expected cross-contamination”: That’s never acceptable in GMP.
(D) talks about side effects: Which are pharmacovigilance and clinical matters, not core GMP concerns.
So (A) is spot on and regulators love precision like this.
Hello Sir,
I have some question.
According to the recently revised EU GMP ANNEX1, it says that a study should be done on the Fh value, does anyone have any experience on how to do this?
8.26 when a thermal process is used as part of the depyrogenation process for any component or prodcut contact equipment/material, validation studies should be performed to demonstrate that the process provides a suitable fh value and results in a minimum 3log reduction in endotoxin concentration.
A hot topic after the EU GMP Annex 1 revision.
What’s the EU Annex 1 saying in 8.26?
If you’re using dry heat for depyrogenation, you must:
• Prove your Fh value (heat lethality specific to endotoxins).
• Demonstrate ≥3 log reduction of endotoxins (i.e., 99.9% reduction).
• Validate it with real thermal mapping and endotoxin challenge studies.
What’s Fh?
Think of it like F0 but for endotoxins, not microbes.
Formula:
Fh = ∑10^[(T–D)/Z] · Δt
Where:
• T = Actual temp
• D = Reference temp (usually 250°C)
• Z = 46.4°C (specific for endotoxins)
How to do it (in 3 steps):
1. Thermal Mapping:
Place calibrated thermocouples in worst-case locations in the depyrogenation tunnel or oven.
2. Endotoxin Challenge Study:
Use vials spiked with ≥1000 EU of standardized endotoxin.
Expose them under normal and worst-case cycles.
Post-process, test for residual endotoxins using LAL.
3. Calculate Fh Value:
Show your validated cycle delivers Fh > 30 (to meet 3-log reduction, assuming D = 10 min).
Pro tip:
If your equipment shows Fh ≥ 30 and your recovery shows <1 EU, you’re golden.
Annex 1 wants science-based assurance & not only “run it for 30 mins at 250°C.”
I am looking to find the time and temperature requirements to destroy the toxin produced by Staphylococcus or similar organisms. These toxins heat resistant but not sure what is the D and z values. Thanks,
The Target: You’re asking about Staphylococcal enterotoxins (SEs); mainly SE-A, the most studied.
These are not your average bugs. They are heat-stable toxins, meaning even after the bacteria die, the toxin remains active.
What does it take to inactivate them?
According to published research (including WHO, FDA, and EFSA sources): D-value at 121°C ≈ 4 to 6 minutes.
That’s the time to achieve a 1-log (90%) reduction. Z-value ≈ 20°C to 22°C
(This means every 20°C increase cuts D-value by 10x)
Temp. Estimated Time for 3-log Reduction
121°C ~15 – 18 minutes
130°C ~5 – 6 minutes
135°C ~1.5 – 2 minutes
Note: These are for purified toxins in buffer. Real-world matrices (protein, fat, pH) increase the resistance.
Key Takeaway:
If you’re designing a decontamination or sterilization process, aim for:
• ≥3-log toxin reduction
• Fh or equivalent thermal input >15 minutes @ 121°C
• Use validated biological indicators or spiked samples if possible
Bonus Insight:
Just because you kill the bug doesn’t mean the product is safe.
Always validate both microbial inactivation and toxin destruction, especially for staph-related risks.
Hi Saket, thanks for your great work! I would like to ask you a question about Microbial Validation if you can kindly help me.
I’ve read in the PDA techinical Report No.3 that ” A minimum of ten challenge units should be employed per challenge. “.
So the question is: there are others possibility of microbial tests during a depyrogenation’s validation process? In particular, there are other sources indicating a minimum appropriate number of challenge for challenge?
Thanks a lot, have a nice day!
Davide
Thanks Davide! Great question and very relevant for anyone validating depyrogenation tunnels or dry heat ovens.
PDA Technical Report No. 3 is indeed a strong reference. It recommends: “At least 10 biological indicator (BI) units per challenge location.”
Why? Because microbial validation is about statistical confidence beyond PASS/FAIL.
So, are there other references?
Yes. Here’s how they line up:
Guidance | Minimum Challenge Units
PDA TR No. 1 & No. 3 | 10 BIs per location
USP <1229> | Aligns with PDA approach
EU GMP Annex 1 (2022) | Doesn’t specify a number but expects “robust validation using worst-case locations”
ISO 11138-1 | Focuses on BI characteristics, not quantity but supports statistical confidence
What does that mean practically?
Use 10 units per challenge location as your minimum unless:
– You’re in early development (feasibility work), or
– Your risk assessment justifies more based on equipment size, airflow complexity, or criticality of load.
Don’t go below 10 for routine validation; you’ll weaken your data set and possibly raise auditor concerns.
Make sure your BI placement reflects worst-case cold spots & not just proper spacing. FDA and EMA expect that logic.
Sir, in depyrogenation tunnel, 250 degree for 30 min, Z value is 46.4 for 1 log reduction.
Total temperature required the 294.4 degree for 1 log reduction.
Then why we validate tunnel at 300 degree insted of 294.4 in pharmaceutical applications?
Plz clear my confusion.
Great question, Anuj. Thinking like a pro.
Even though 294.4°C theoretically gives 1-log endotoxin reduction, we validate depyrogenation tunnels at 300°C because:
– Regulatory safety margin: 300°C is a conservative standard backed by literature and industry practice.
– Process variation: Tunnel temperatures fluctuate slightly, so 300°C ensures robustness.
– Fh consistency: Most tunnels are designed for ≥15 Fh at 300°C for ≥3-log reduction.
Remember, validation is science plus compliance assurance. 300°C builds confidence.
Sir, Thanks for your effort,
I Have a question please, I need to know what the minimum value of Fh and Fd to Achieve a successful dry heat sterilization and depyrogenation,
what I realized that the ideal is to achieve Fh 32 min at 170 C and Fd 30 min at 250 C, what if I change the temperature how could I Calculate the required time to achieve a successful Process?
Once I was doing a validation for an oven in one of the companies, they set the Oven at 185 C for 2 hrs. and they considered this run as depyrogenation study and the result of Fd was 4.78 is this is failed run?
As an example, should I increase the time to achieve a 30 min of Fd to be success with taking in consideration increasing time to prove my understanding of doing a success depyrogenation run?
Thank you.
What Fh and Fd Mean in Validation
• Fh is for dry heat sterilization (killing microorganisms)
• Fd is for dry heat depyrogenation (destroying endotoxins)
For:
– Sterilization (Fh): Target value is ≥15 mins at 170°C. Purpose: Ensures microbial kill (z=10°C)
– Depyrogenation (Fd): Target value is ≥30 mins at 250°C. Purpose: Ensures 3-log endotoxin reduction (z=46.4°C)
Now, 2 hours at 185°C will not give you enough depyrogenation. You would need many more hours to reach equivalent lethality.
So yes: Your Fd of 4.78 is too low for a valid depyrogenation run.
Either increase the temperature or increase time to achieve Fd ≥30. Hope this helps.
Dear Sir,
Role of D value in FH calculation in Depyrogenation tunnel and Dry heat Sterilizer temperature validation where in both cases I am using endotoxin as a challenge?
I am using Kaye Validator for calculation of FH value During exposure time:
For DHS,
Base Temperature is 250 C. Dwell time is 30 Min. D value I put 4.99 Min.
Acceptance criteria is FH not less than 30 Min.
But, I am not getting required FH value.
Sir, where is the problem I am not able to find it.
For Depyrogenation Tunnel,
Base Temp is 250 C. Dwell time is 3 Min. D value is 4.99 min.
Here also not getting required Fh value? Where is the problem?
One more thing. In both cases I put Z value as 46.4 C and getting the required temperature i.e in case of DHS temperature is greater than 250 C and for depyrogenan tunnel greater than 300 C.
Sir, Please reply.
Great question.
Fh calculation depends on time, temperature, D value, and z value. You’ve used the right z (46.4°C) and D (4.99 min); but here’s the catch:
Dwell time alone isn’t enough if:
– Your actual temp. isn’t maintained uniformly
– There’s too much fluctuation during exposure
– You’re using short exposure like 3 min in the tunnel (which often needs temps ≥ 300°C)
Try this:
1. Recheck actual temp mapping at the coldest spot
2. Ensure stable dwell temp & not just peak
3. If needed, increase time or temp to meet Fh ≥ 30
Dear Sir,
I have depyrogenation tunnel running on 330 degree Celsius. Dwell time is approximately 8 minutes. What should be z value for it?
Great question. For depyrogenation at 330°C, the commonly accepted z-value is 46.4°C – based on literature (Tsuji & Harrison). This z reflects thermal resistance of bacterial endotoxins (mainly from E. coli).
Even though your temp is high, stick to 46.4°C for Fh calculation unless you have organism-specific thermal resistance data.
Hello Sir! What will be the depyrogenation time for a tunnel operating at 335 deg.C?
At 335°C, you’re well above the base temperature of 250°C typically used for depyrogenation Fh calculations.
If you’re using a z-value of 46.4°C and targeting an Fh ≥ 30 minutes (equivalent to 3-log endotoxin reduction), the actual dwell time needed drops significantly, likely under 5 minutes depending on heat distribution.
But don’t guess. Always confirm with thermocouple mapping + endotoxin challenge to validate performance.
Good morning Saket!
First of all, thank you for your clear and insightful article!
I have a question about a “reverse problem”.
We operate depyrogenation of glass vials in a tunnel at 350° C. As we want to decrease the cycle time, may I estimate the minimum cycle time by using the Fd equation?
I tried with the following set:
Tref=250 C
Ttunnel =320 C (lowest stable recorded temperature)
z=46,4°C
and I obtained a delta t=0,93 min.
Of couse this could not be the cycle time since there the heating time (from 20C to 320C must be taken into account. But if for example heating time is 2 min, it is correct and safe to affirm that an Fd=30 min is reached in 3 minutes? I suppose that it could be greater since in the heating phase when T tunnel >250 accumulation of Fd starts.
Thank you!
Great question and smart you’re reverse-engineering Fd with real numbers.
You’re right: once Ttunnel > 250°C, you’re already accumulating Fd.
So yes, cycle time = heat-up time + effective dwell time.
If your lowest recorded T = 320°C, z = 46.4°C, and you’ve got 2 min to heat + ~1 min to hit Fd 30:
You’re likely safe on paper.
But regulators won’t accept math alone. Confirm with actual endotoxin recovery and mapping to back up your model. That’s your safety net.
Sir, please tell me how to calculate sterilization hold time of depyrogenation tunnel…
Use this formula:
Fd = ∑10^((T−Tref)/z) × Δt
Where:
• Fd is depyrogenation lethality (min)
• Tref is 250°C
• z is 46.4°C
• T is real-time temp
• Δt is time interval
Then? Reverse-calculate how many seconds above 250°C are needed to reach Fd ≥ 30 minutes.
Hello sir, great explaination
I have few questions,
1) How do we identify the z value for a depyrogenation process?
2) What is the mininum FD to consider as complete depyrogenation?
Thank you
Great questions.
1. Z-value for depyrogenation is typically 46.4°C: Derived from studies like Tsuji & Harrison. It reflects how fast endotoxin inactivation accelerates with temperature.
2. Minimum Fd to qualify as effective depyrogenation is 30 minutes at 250°C. That equals a 3-log reduction of endotoxins – the global gold standard.
Remember: Always validate with biological indicators like LAL to confirm it’s not just math, it’s real-world kill.
Hello Saket,
Thank you for an interesting practical article.I am hoping you can help me with a Dry Heat Sterilization question. An overkill DH Steri process was validated at 150C at 4 hours (half cycle). Someone changed the cycle to 130C at 6 hours (half Cycle). I have been away from these calculations for a long time- I know I should be able to show equivalency of the two temp/time combinations to show we still have a 6 log reduction for the half cycle. Can you help?
Hi Saket,
Your answer confirmed my results and I was hoping I was wrong!
The cycle is in the process of being validated at the 130°C for 6 hours. I am keeping my fingers crossed that it passes the Microbiological PQ.
The people changed it do not understand micro or sterilization and I was brought in to help fix the problem.
Thank you again for your response.
We are in similar situation. We make color cosmetic powder and we need to lower our bioburden however our product has heat stabilty of 140 C. We ran 115C for 6 hours and it didnt work in removing TAMC, Gram+ and Gram- so we wanted to try 130 for 6 hours. Any thoughts on the feability of if it will work?
Glad you find the article helpful, Barbara! Surely I can assist. Let me summarize the findings for you here.
1st Case: 150’C for 4 hours (240 minutes) — Fh value = 24 mins. __ Validated
2nd Case: 130’C for 6 hours (360 minutes) — Fh value = 4 mins. ___ Not-validated
Ideal Case: 170’C for 32 minutes — Fh value = 32 mins.___ Ideal
The difference is clearly visible. 130’C for 6 hours doesn’t seem to be working for you.
I recommend you in this case:
1) Resume your half cycle to 150’C for 4 hours which was already validated. OR
2) Go with the ideal case mentioned above. OR
3) Try to validate it for 130’C for 6 hours (I don’t feel it will help).
Also, investigate –
1) Why the cycle parameters changed and on what assumptions?
2) Impact on the product quality for already processed batches under 130°C at 6 hours cycles.
3) Document the deviation and conclude your CAPA accordingly.
Theoretical Requirement: 170°C at 32 min. of sterile hold time.
Can you show me to the documentation about this?
Dear Saket,
thank you for this valueable article for the newbies in the field.
I’d like to etent Susu’s question for the reference to the Theoretical requirements also for steam sterilization and depyrogenation.
The (base) temperatures are not in line with E.P 5.1.1.
We also need to clarify that the recommended durations are for the full cycle and not only for the sterile/depyrogenation hold time, else the would exceed regulatory requirements by far.
Thanks in advance for your clarification!
Best regards,
Robert
Great catch, Susu! The 170°C for 32 minutes target is based on achieving an Fh of 30 minutes using a Z-value of 10°C; commonly referenced in dry heat sterilization validation.
For documentation, check:
• USP <1223> (Validation of Dry Heat Sterilization)
• PDA Technical Report 1
• EN ISO 20857:2020 (Sterilization of health care products by dry heat)
These standards lay the foundation for Fh-based cycle design.
Great points, Robert, and I appreciate you raising them.
You’re absolutely right:
The temperatures and durations often quoted (for example, 121.1°C for 15 minutes or 250°C for 30 minutes) are industry practices, not strict pharmacopeial mandates.
The European Pharmacopoeia 5.1.1 provides minimum validated process conditions for sterility assurance, not total cycle durations.
What we usually quote includes total exposure hold, not the full cycle (including come-up and cool-down phases). That’s an important distinction.
Thanks again for the clarification. It’s a reminder that context is key when applying regulatory benchmarks.
How to find FH value at 180 degree celsius and having hold time 1 hour. And what will be the z value for that??
To calculate Fh at 180°C, you’ll need the reference D-value (typically at 250°C) and the z-value for depyrogenation, which is generally 46.4°C. Use the standard Fh formula:
Fh = ∑10^[(T – 250)/z] × Δt
In your case:
• T = 180°C
• z = 46.4°C
• Hold time = 60 min
Since 180°C is far below 250°C, the Fh contribution will be very low, almost negligible. You’ll need much higher temperatures to achieve an Fh of 30 or more.
Sir,
Please tell that, why we consider 20°C as Z value in dry heat sterilization (Fh) while in depyrogenation 46.4°C?
Great question. The Z-value represents the temperature change needed to cause a tenfold change in the D-value. For dry heat sterilization, a Z-value of 20°C is used based on microbial inactivation kinetics. In depyrogenation, 46.4°C is used because it reflects the thermal resistance of endotoxins, not microbes. The difference comes from what you are trying to kill – microbes vs pyrogens and how each reacts to heat. These Z-values are based on experimental data and industry consensus.
When calculating with a different program, two values were obtained: Fh=13.78(min) and Fh=0.07(min).
0.07(min) could be obtained in the same way as your writing, but I have no idea how 13.78 is calculated. Do you happen to be able to seek advice?
You’re right to question that 13.78 min value. If both results are for the same time-temperature profile, one of them likely used the wrong Z-value or formula base. Most Fh calculations use a reference temp of 250°C and Z-value of 20°C. Recheck:
1. Was Z = 20°C in both?
2. Was the full exposure time used?
3. Any temperature overshoot?
Mismatch usually comes from input assumptions. Always check the math before trusting the output.
Sir plz tell me the Z value for 220°C at 90 min. in DHS.
Z-value isn’t based on time or temperature alone; it’s a fixed constant for the type of microbial challenge you’re targeting. For dry heat sterilization (DHS), standard Z-value is 20°C, based on typical thermal resistance of bacterial spores. Use this with your time-temp data to calculate Fh.