Types of Filter Integrity Testing

Filter integrity testing ensures your product stays contamination-free. It checks the integrity of filter membranes, disk filters, and cartridges – so nothing unwanted gets through.
The machine that does this? A filter integrity tester. It must comply with Class B and Digital Devices under Part 15 of FCC regulations and typically operates on the differential pressure principle.
But here’s where it gets interesting:
These machines come with built-in data management systems, making it easy to handle test records and generate reports – so compliance and traceability are never a headache.
Sterilizing filter performance comes down to one key factor: bacteria retention below 0.22 µm.
To prevent overload on a single small-sized filter, multiple filters of different sizes are used in sequence – stepping down the particle load efficiently.
The first filter has a 0.45 µm size followed by 0.22 µm. Later, they get sterilized with steam for another batch process. These filters require filter integrity testing both before and after use if tested non-destructively.
The two cases to perform filter integrity testing are:
- New Filters
- Filters that require re-use
In the first case, filter manufacturers themselves perform the testing along with certificates and documentation. If so, it is not mandatory for drug-makers to test new filters. However, they may do to have more confidence.
Meanwhile, the second case often requires testing to check how intact the pore size of the membrane is. Testing the differential pressure helps to assure the integrity of the filter. Agar plates re-check that no bacteria crossed the 0.22 µm filter.
Table of Content
Classification of Filter Integrity Testing
The FDA set forth guidelines and requirements to conduct and document filter integrity testing.
The testing is classified into two categories:
- Destructive Testing
- Non-Destructive Testing
Destructive Testing
Destructive Testing is by and large carried out at the filter manufacturer’s location from each lot as per their predefined sampling plan.
Destruction of filters takes place to ensure that no bacteria can pass through the membrane. Simply put, filters retain bacteria.
This testing complies according to the method outlined in ASTM F838-05 (formerly ASTM F838-83), Standard Test Method for Determining Bacterial Retention of Membrane Filters Utilized for Liquid Filtration.
Both Health Industry Manufacturer’s Association (HIMA) and FDA have defined the retention of the challenge bacteria level to be 107 per cm2.
The FDA considers a filter as “Sterile” when it retains the challenge of bacteria Brevundimonas Dimunita (ATCC 19146) at least 1×107 per cm2 of filtration area. For more information, see an insight into membrane filter validation.
Non-Destructive Testing
Filters used in the previous batch may be damaged or distorted ultimately posing questions on their integrity for the past and coming batch.
Reusing a filter adopts non-destructive testing where it is checked for its intactness and quality for the next batch.
If filter integrity testing fails, the previous batch may need re-testing/processing as per suitable immediate corrective action. The filter also becomes useless and hence discarded.
The following are the different methods for non-destructive filter integrity testing:
- Bubble Point Test
- Diffusion Test
- Pressure Drop Test
- Water Intrusion Test
These tests were developed to physically make sure the integrity of a filter cartridge. Each test has its own significance and working mechanism explained below.
Bubble Point Test
This test works on the principle of capillary action and surface tension.
Liquid when passed through the filter, fills inside the pores with the help of surface tension and capillary action.
The minimum amount of pressure required to remove the liquid out from the pores helps measure the pore size. The pressure is applied accordingly.
Nowadays testing units have pre-loaded automated sequences for each test.
The bubble point test goes like this:
- Filter is wetted with suitable solvent (‘water for hydrophilic filters’ and ‘water+alcohol for hydrophobic filters’) uniformly.
- Pressure is applied to the upstream of the filter. The amount of minimum pressure expected from the recommendations provided by filter manufacturers.
- Pressure increases slowly until bubbling occurs downstream.
If bubbling appears at a lower pressure than the set pressure, the test is considered a fail. The pressure at which bubbling occurs downstream of the filter is its Bubble Point.
Possible reasons for failure:
- Incorrect installation of the filter inside the housing
- Filter wetting is non-uniform
- The unsuitable solvent used for wetting the filter is causing compatibility issues due to different surface tension than recommended or;
- Filter failed integrity due to deformation
Once we get the bubbling point, the diameter of the pore is calculated by:
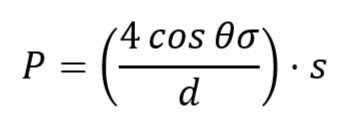
where,
P – min. pressure when bubbling occurred
d – Pore dia.
θ – Contact angle between solid and liquid (this angle formed because of surface tension and capillarity)
s – correction factor
σ – surface tension
Don’t forget to maintain unit consistency.
Diffusion Test
This test works on the principle of Fick’s Law of Diffusion, where the rate of diffusion is directly proportional to the membrane’s surface area.
The effectiveness of the diffusion test depends on the downstream gas flow rate, unlike the bubble point test where pressure was the key aspect.
Typically, the pressure used to perform this test is 75-80% of the bubble point of that membrane. The manufacturer provides requirements/specifications for this test.
The procedure goes like this:
- Filter is made wet uniformly with a suitable solvent.
- Pressure is applied upstream of the membrane, gradually increased up to the recommended value and allowed for stabilization.
- Downstream gas flow-rate is measured with the help of a suitable flow meter for 60 seconds.
If the value of the gas flow-rate comes higher than the recommendation, the test failed.
Possible reasons for failure:
- Incomplete wet membrane
- Insufficient stabilization
- Filter failed integrity due to damaged pore size
Verification of the test is done by using,
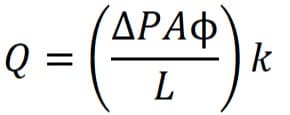
where;
Q – Diffusional flow
A – Membrane surface area
L – Effective Length
ΔP – Differential Pressure
Φ – Porosity of the membrane
k – Diffusivity
Again, don’t forget to maintain unit consistency. This test is recommended when handling high-surface-area systems starting from 1.5 m2 onward.
Pressure Drop Test
While dealing with different housing arrangements, bubble point or diffusion tests becomes ineffective.
Then, pressure drop tests are performed on the automated filter integrity testing units.
Because this test is performed upstream of the assembly, the downstream manual operations get eliminated.
As manufacturers do not specify the exact test requirements, this test relies on system volume data. They measure changes in upstream pressure because of diffusion via the application of an appropriate and accurate pressure gauge.
Maximum allowed pressure drop:
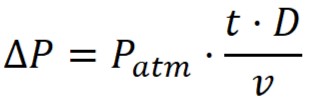
where;
D – Rate of Diffusion
Patm – Atmospheric Pressure
V – Volume of system upstream
t – time
ΔP – Pressure Drop or Decay
Water Intrusion Test
This test is typically performed to detect the quality of defects of hydrophobic filters. These filters are installed as air vent filters.
Here’s the article that explains water intrusion test requirements.
Typical Arrangement
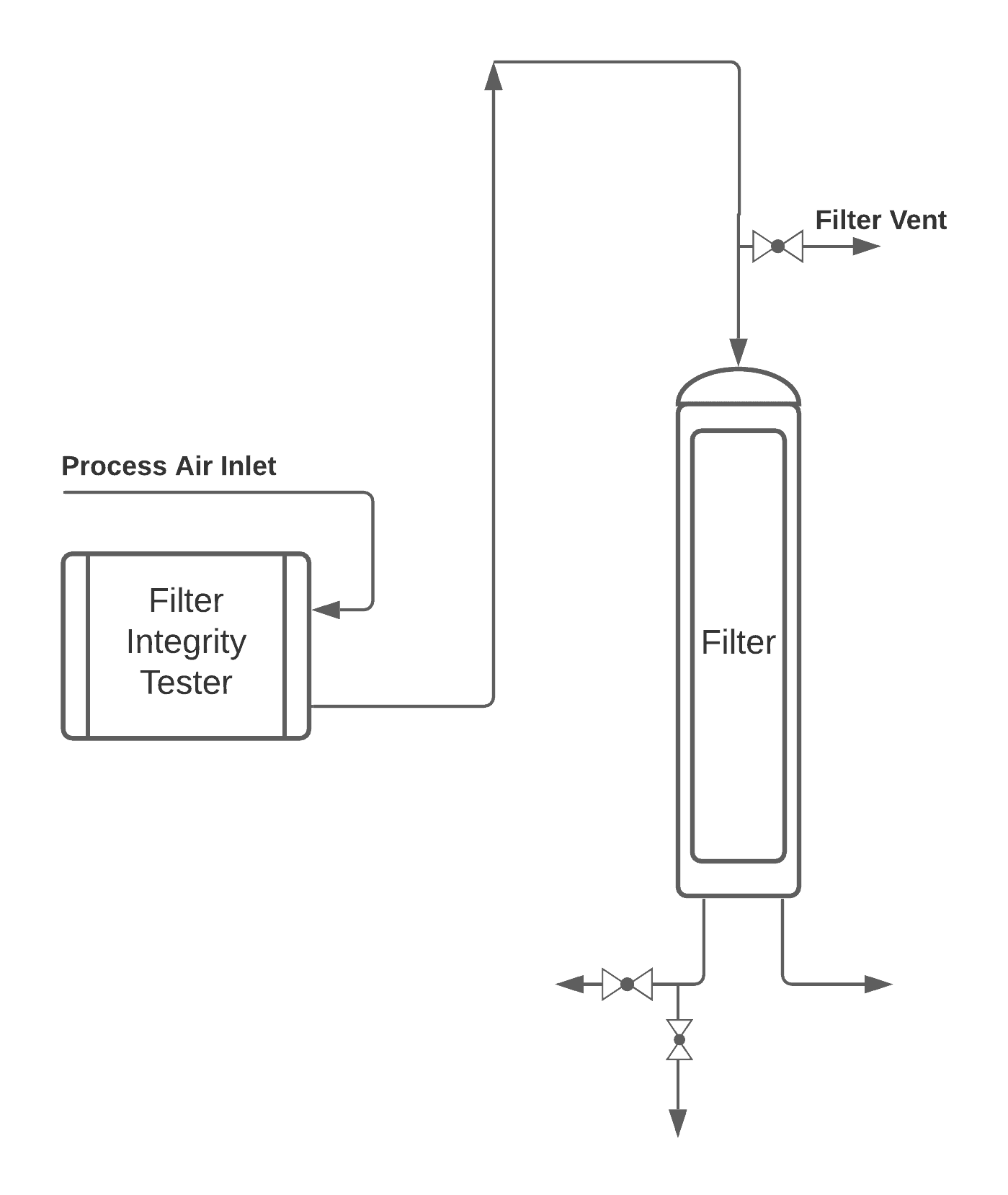
Generally, common components installed on the testing unit are housing, HMI, printer, tubing, alarms, TC Clamp connections, etc.
Proper connections at the inlet and outlet prior to starting the test sequence must be checked before initiating.
Check the proper recipe is set, loaded, and ready to execute.
Automation Aspects for Filter Integrity Units
The reliability of the testing unit components depends on their MoC. Hence, compatibility with the laboratories and commercial manufacturing environments is important.
The manufacturer takes care of that part, including automation hardware.
To prevent mishandling during emergency conditions, alarms and interlocks should be considered.
The system prevents any unauthorized access to recipe modification, start and stop sequence, and other functions.
An audit trail is another requirement that captures all the activities any user performs. Secure backup and restore procedures are also included in the automation package.
GxP Requirement for Filter Integrity Units
- 21 CFR Part 11, Electronic Records, and Signatures
- GAMP 5 A Risk-based Approach to compliant GxP Computerized Systems
- BS EN 60204-1, Safety Guidance and Recommendations on Electrical Equipment
- Certificate of Compliance
Some test units produce paper-based records storing no data electronically. Such units don’t fall under the requirements of 21 CFR Part 11. Also, the application of such units is not designed for cGMP-regulated environments.
For testing units requiring compliance with FDA 21 CFR Part 11, the validation plan should cover procedural and technological control.
Consider the following points while complying with 21 CFR Part 11:
- Generation, Modification, and Maintenance of Electronic Records
- Audit Trails for all the Electronic Records
- Equipment Qualification and Test Method Validation
- Training of Concerned Personnel for 21 CFR Part 11
- Electronic Signatures
- User Management
- Data Export in the format suggested by FDA
- Human Readability for defined retention time
Filter Integrity Test: FAQs
What is filter integrity test?
Tests carried out to verify and assure the quality and readiness of the filter membrane regarding the regulatory requirements are called filter integrity tests. If failed, shows that the filter is no longer re-usable, and also the previously filtered batch needs inspection.
How does a filter integrity tester work?
As mentioned previously in this article, two basic classifications include Destructive and Non-Destructive testing. Non-Destructive tests include testing based on Bubble Point, Diffusion, and Pressure Drop tests as suitable to the requirements.
What is bubble point test for filters?
This test works on the principle of capillary action and surface tension. Liquid when passed through the filter pores fills inside the pores with the help of surface tension and capillary action. Pressure is applied to measure the diameter of a pore. The minimum amount of pressure required to remove the liquid from the pores helps in measuring the pore size.
What is water intrusion test?
This test is typically performed to detect the quality of defects in hydrophobic filters. These filters are generally installed as air vent filters. Water with pressure penetrated inside the filter upstream to compare the outcomes with the manufacturer’s specification limits.
What does bubble point mean?
The minimum amount of pressure at which the first bubble of liquid comes out from a pore of the membrane, known as the bubble point for that filter.
What does a 0.22 micron filter remove?
The diameter or size of the pore is uniformly maintained on the filter membrane. We consider this size one of the smallest in the industry to eliminate the finest-sized particles. This is effective in removing bacteria and not viruses.



The details given in the data is quite good.
But i had a doubt that whether cartridge filter which is being used in PW Generation system (5 micron and 50 microns). so when the filter is being discarded, it is required to do the filter integrity test for the filter or not?
Great question. For prefilters like 5 and 50 micron cartridges used in PW generation, filter integrity testing is not mandatory as it is for sterilizing-grade filters. Instead, visual inspection, differential pressure monitoring, and defined replacement frequency are standard practice. Integrity tests are reserved for critical final filters where sterility assurance is required.
Dear Saket,
If I just have information of Flow rate and pressure drop, no integrity testing information. how I can calculate and set up integrity testing for this filter. I have a machine for integrity testing (IT5 of Merck)
“Flow rate and pressure drop: Sample met maximum pressure drop of 6 psi (410mbar) at 10 gmp ( 38L / min) per 10in. equivalent with water at 23 deg.C”
Thank you so much!
Thanks for the detailed question.
What you’ve shared is hydraulic flow resistance data – useful for performance, but not sufficient for setting integrity test specs like bubble point, diffusion, or pressure hold.
To set up integrity testing on the Merck IT5, you need:
1. Filter membrane type (e.g. PTFE, PES, PVDF)
2. Pore size (e.g. 0.22 μm)
3. Manufacturer’s validated test limits for:
• Bubble point
• Diffusional flow or pressure decay
• Test pressure
Next steps:
• Contact the filter supplier for certified integrity test parameters.
• Don’t derive limits based on pressure drop alone – it doesn’t correlate with microbial retention.
Great article. Auditors are expecting filter integrity validations and sending used filters for filter integrity testing.
Is it just to use the smallest bacteria at 0.22 micron and it’s able to filter it?
Thanks
Thanks for the thoughtful comment.
Filter integrity testing is not just about trapping any small bacteria; it’s about proving that your sterilizing-grade filter (e.g. 0.22 μm) consistently retains Brevundimonas diminuta, the gold standard challenge organism sized at ~0.3 μm by 0.17 μm in flexible form.
Regulators expect:
• Pre- and post-use integrity testing
• Validated test methods (bubble point, diffusion, pressure hold)
• Data traceability to the filter’s retention claims
Integrity = performance proof, not assumption.
And yes, always test used filters; it’s your best defense that sterility wasn’t compromised after the run.
Nice way of explanation
Thanks!
Great efforts Saket!!!
Loved your articles
Keep up the good work 👍🏽
Hey Sagar! Really appreciate your words 🙂
Helpful knowledge. Works smoothly as per interview aspect.
Thanks!
Hi.. Helpful article for overall understanding. Can yo share the calculator for these tests as you shared F0 value calculator. That would be of great help. Thank you.
Thanks for the feedback!
Yes, just like the F0 calculator, a Filter Integrity Calculator can definitely be built to simplify:
• Bubble Point threshold checks
• Diffusion flow limit validation
• Pressure hold time conversions
• Test gas vs liquid corrections (if needed)