Important Aspects of Good Documentation Practices (GDP) and 9 Principles of ALCOA+

Document what you do and do what you document.
In the life-science realm, almost everyone must have heard this saying.
GDP is the one of the highest contributing factor in the firms receiving the warning letters and even market bans.
Major consequences of not ensuring GDPs:
- Regulatory restriction for your product distribution.
- Difficulty to explain the auditors on how will you repair the broken data.
- Damage to market reputation.
- Customer distrust.
- Financial losses and so on…
These are the continuing disgrace to the well-established organizations.
This happens mostly due to the following reasons:
- Hard to follow procedures written by senior staff that force on ground staff to deviate their work.
- Lack of training and assessment.
Solution?
- Easy to follow procedures written by the staff that actually works on the ground.
- Frequent training exercises and evaluation.
The framework in which such exercises are included is called Good Documentation Practices (GDP).
GDP guidelines tells us how to record the raw data and make it meaningful.
The more meaningful your documents are, the less you speak during audit inspections.
Let us see in detail.
Table of Content
What is a GxP Document?
GxP Document is a document identified as critical to comply with the current GxP regulations
For instance, Good…
- Manufacturing Practice (GMP)
- Laboratory Practice (GLP)
- Documentation Practice (GDP)
- Distribution Practices (Again GDP)
- Clinical Practice (GCP) etc.
Good Documentation Practices have a generally practiced set of characteristics described further.
ALCOA and ALCOA+
Most professionals working in the life-sciences are aware of ALCOA (explained further) principles of Good Documentation Practices for data integrity.
And, some industries are advancing from the ALCOA to ALCOA+.
Overall, they’re the heart of Good Documentation Practices. Because, they help to produce and maintain quality records.
Attributable (A):
Attributable means the document is capable to determine the origin and history of the performed activity.
The document is also capable to refer to the individual responsible for the creation, maintenance, or handling of the record.
When investigating critical situations, this helps in traceability. Thus, sometimes Attributable is also termed as Traceable.
Legible (L):
Legible means the document is meaningful i.e. Readable and Relateable.
Contemporaneous (C):
Contemporaneous means Parallel, Simultaneous, or Concurrent. The document recorded simultaneously to the performed activity, considered as a contemporaneous entry.
Data shall be recorded when the activity completes (includes time and date stamps for electronic records).
Original (O):
Any form of quality record such as electronic, paper, magnetic, or optical is considered as Original.
The original term refers to the availability or presence of data in its crude form, whether it is in paper or any other but in original form.
In simple words, the record maintained at the source of its generation.
However, prints generated on some papers may fade away over time. For that, photocopies shall be immediately made to ensure the data is not lost in the future.
Accurate (A):
The document that is consistent, factual, and recorded as it is.
Meaning, no chance of question regarding the content of the document.
Refinement to ALCOA, few other characteristics critical to data integrity were identified by the quality experts and termed as ALCOA+.
The Pluses (C-C-A-P)
Complete:
Data with necessary information providing a complete description of documented events without any gaps such that following that description, the events can be reconstructed or repeated.
Chronological:
The document containing information consistent with the sequence of the events.
Available:
Data must not merely exist, it must be accessible all the time.
Permanent:
All quality records must follow the appropriate record requirements for handling, storage, and disposition whether a record is paper or electronic
All quality records (paper or electronic) must be retained, handled, and disposed as appropriate to the requirement.
Common Guidelines for Good Documentation Practices
Apart from the above core GDP characteristics, here’re the other important guidelines.
- Recording the entries before performing the activity or at a later time or backdated is strictly prohibited. Write as you do and do as it’s written. This ensures strict adherence to the established procedures.
- The recorded activity must have the sign and date of the individual who performed it.
- Legible or readable data is archived up to the document life. For handwritten entries: Enter all information in non-erasable, permanent, non-water-soluble ink. Using a pencil is prohibited.
- Short-hand notes or vague statements are not allowed.
- Use hard-bound controlled logbooks with numbered pages for easy traceability.
- Take a photocopy for the data printed on thermal paper (with original affixed) and sign and date both.
- Transcribe information in case of torn or damaged original paper with proper identification, issuance, and keep original copy attached to the transcribed page for evidence.
- Address all abbreviations in the record.
- Do not leave blank spaces in the record and fill in the blanks as “Not Applicable” or simply “N/A” with a single cross line diagonally, sign, and date.
Example of Striking Not Applicable
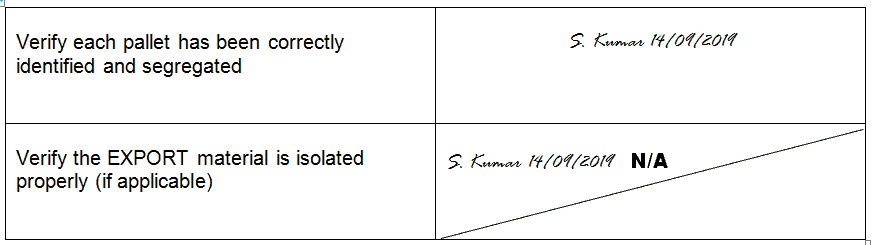
- Provide an explanation for intentionally left blank spaces if to be filled later.
- Use consistent Date and Time format throughout the document or record. Date format in all numbers is acceptable, provided the same is captured in the document itself. Date format may depend region-wise. For example,
Europe: dd/mm/yy or dd/mm/yyyy
US: mm/dd/yy or mm/dd/yyyy etc. - Time format may include 12-hour format or 24-hour format. If the time is recorded using the 12-hour format, then time must include the identifiers AM or PM (example: 8:00 AM, 9:00 PM). Any format of AM or PM (e.g. AM, a.m.) is acceptable as long as its meaning is clear in context. In the 24-hour format, 00:00 is midnight and the remaining hours are in sequence from 1 to 23 (e.g. 22:30 hr, 08:20 hr, etc.).
Round off values
- No round-offs are allowed for observed values (such as individual temperature probe reading) recorded.
- Round off is not performed till we calculate the last value.
- If the first figure is ≥ 5, increase by 1; the digit in the last place maintained. Example: 145.366 = 145.37 or 145.365 = 145.37
- If the first figure is < 5, it maintains the previous digit. Example: 145.364 = 145.36
Data Correction
- Never erase original entries.
- Apply only a single strike on the entry error to keep it legible and then make corrections.
- Cross the entire entry through, even though only a portion is incorrect with sign and date. (e.g. cross out the entire number or word, not just one number or letter).
- Re-approval is required if corrections made to the previously approved document. Ensure the same function(s) carry out the re-approval activity.
- Only doer or his/her supervisor can correct the data with explanation, sign, and date. Where space is the constraint, the correction should clearly link to the corresponding reason at the bottom of the page.
- If multiple corrections on the same page, use the sequential numbering method for proper identification with each sign and date individually.
- Do not use correction fluid (whitener), tape, blade, or any other method which shows intentional data hiding.
Data Tampering
Any act of hiding data may in the form of fabrication, intentional misplace, falsification, alteration, or deletion is Data Tampering.
Willful false report or misleading the data is not allowed including at least:
- Backdated entries
- Deliberate omission or manipulation of data that hides the event
- Sharing account credentials across team members
- Entries for the activities performed by another associate
- Intentionally keeping correction fluids in plant or rough paper works etc.
- False signatures
- Fabrication of records (creating reports for which activities yet not performed)
Managing Annexes
- Any supporting data is attached to the record must be labeled with proper identification and label.
- Enlist attached appendices documented in the main report/record as applicable.
- Use page number formatting as “A of n”, where A=page number while n=total number of pages. For referencing annotations from regulatory bodies, page numbers are needless.
- A best practice is to apply the header (the title of the document) and footer (page numbers) to a PDF file if attached as Appendix. Wherever possible, keep attachments in a non-editable format with watermarks.
Signatures
GxP documents require a Signature with a date for identification and traceability in the future course of the review.
Further, the significance of each signature according to Good Documentation Practices is mentioned below:
- Qualified Personnel: Any personnel signing any document or record is trained on that particular activity or entitled as Qualified for authorizing or certifying the document or record. Signature in the quality document indicates the accountability for what is contained in the document.
- Performed By: A personnel executing an instruction, operation, or calculation. Sometimes, the performer is also termed as “Doer” in which case “Performed By” turns into “Done By”.
- Verified By: A personnel verifying the activity performed by other personnel. Verification includes making sure or demonstrating the activity performed is true, accurate, or justified.
- Reviewed By: Conformity of completion, compliance to action/s with applicable specifications or requirements.
- Approved By: Conformity to complete review for action/s as per the requirement with all necessary signatures captured and issues addressed through GxP procedures if any.
As per the above philosophy, it does not imply that the performer is not accountable for verification, review, and approval. Moreover, it means the performer has also read, understood, verified, reviewed to the best of his/her knowledge, and then signed.
Ensure use of electronic signatures as per requirements (generally Computer System Validation CSV procedure). Wherever required, a quality review must include raw data, useful metadata, and audit trails.
Missing Data Management
During a review or any point of time, if anyone notices that the data from the record is missing, it can be added if:
- Conformity to the existence of additional data is verifiable
- Responsible function or immediate supervisor provides the required data
- Traceability of who added the data is available
- Additional data is kept in the records with the old data
Record Retention Policy (Storage, Archival and Retrieval)
Apart from the above things, there must be a policy for record management including record retention period, archival methods, and retrieval requirements.
This ensures that the industries follow Good Documentation Practices till the termination of the record.
Recorded data in the form of either hard copy or electronic data must follow the requirements as per applicable local procedures company-wise.
Conclusion
The GDP procedures not only apply to day-to-day commercial manufacturing but also to process validation, cleaning validation, and other quality tools employed in drug manufacturing.
Here are some of the common definitions useful for better understanding of the GDPs.
Definitions
- Audit Trail: Sequential record of computer or controller system activities that enable reconstruction or examination of events or series of events from start to end.
- Controlled Document: GxP document under defined procedures and governed by life-cycle management, including distribution within the organization.
- Data: Suitable representation of facts or instructions for communication or processing information by humans or systems.
- Data Integrity: ALCOA+ provides data integrity i.e. genuineness of the data.
- GxP: Critical documentation necessary to maintain compliance with current GxP regulations such as Good Manufacturing Practices (GMP), Good Clinical Practices (GCP), or Good Laboratory Practices (GLP), etc.
- Meta-Data: Data capable of attributing personnel or meaningful information. Examples include Creation Date, Modification Date, Visual References, or something meaningful.
- Raw Data: Data kept in its originally generated format
- Traceable Data: Able to determine history or origin and tracked to individual responsible for monitoring or recording.
- Good Documentation Practices: Document handling, storage, archival, retrieval, retention, and disposal followed considering trace-ability, ALCOA+ principles, and without hampering data integrity.
Here’s a great summary of GDP.
FAQs
What is the purpose of good documentation practices?
When Auditors challenge the integrity of any activity performed in a healthcare facility, raw data and records assure ready reference and traceability. In any healthcare facility, an activity that does not have a documented record suggests that activity is not performed i.e. question mark on product quality. Hence the good documentation practices are a set of guidelines that help in assuring product quality.
What is good documentation practices in pharmaceutical industry?
Guidelines stating procedures for documenting any activity either on paper or electronically within the manufacturing premice, Truck to Truck and Gate to Gate. These guidelines describe the critical aspects of any document such as Attributable, Legible, Cotemporaneous, Original, Accurate, Complete, Chronological, Available, and Permanent.





Is using a stamp for the “N/A initial and date” on a blank or unused space okay?
Sorry, didn’t understand your exact concern. But if you are talking about the stamp usage instead of manual entry for N/A, you will still need to manually put initial and date.
Can u please explain equipment qualification….
Hi Alex, here is an article for your knowledge addition. This will definitely help you understand about the equipment qualification and process validation as a whole.
https://pharmagxp.com/quality-management/process-validation/