Developing Automated In Situ Filter Integrity Testing
Filters are commonly used in many pharmaceutical processes which are qualified and validated to retain bacteria, mycoplasma, or viruses.
In a GMP process and validated environment, these filters must be routinely tested for integrity as the end user’s method to confirm the structural integrity of a filter before and after use.
Automated in situ filter integrity testing is done to assure the filter integrity without displacing or removing the filters from process equipment. In simple words, “at its original location”.
Typically, hydrophobic filters are subjected to automated in situ filter integrity testing. Hydrophobic filters are non-product contact filters and are often used over an extended period.
Whereas, hydrophilic filters are typically discarded after a single product batch has been filtered. Therefore, hydrophobic filters require multiple integrity tests compared to hydrophilic ones.
Filter integrity can be determined by any of the following four test approaches:
- Forward flow integrity test
- Bubble point test
- Pressure hold test
- Water intrusion test
Hydrophobic filter medium prevents the bulk flow of aqueous-based product through the filter at differential pressure below the intrusion pressure of the medium, while permitting the free passage of gas through the filter.
Forward flow integrity test, Bubble point test, and pressure hold test methods require the use of wetting agents such as alcohol or any chemical mixed Water wetting fluids, etc. to thoroughly wet the filter membrane before the filter integrity testing. These tests are advised to perform off-line to prevent contamination from wetting fluids to the process equipment or downstream side of the filter membrane.
Water intrusion tests can be performed In-situ using a water flow mechanism. This method is widely used to develop the automatic In-situ filter integrity in the drug process equipment since it eliminates the use of wetting agents and does not require the penetration of filter membrane by wetting agents.
Table of Content
Water Intrusion Test
The water intrusion is the volume of water that penetrates (intrudes) into the structure of a hydrophobic membrane at a given applied pressure and time.
The water intrusion test has been devised to eliminate the need for solvent wetting with wetting agents or containment such as isopropyl alcohol or ethanol to integrity test hydrophobic PTFE membrane filters.
The use of IPA, especially if attempting to test multi-round systems can also cause a safety issue due to flammability or any possible chemical reactions.

Water intrusion test results can be expressed in two ways i.e., water flow and water intrusion.
- Water flow is a measure of the actual water volume change at test pressure, which is ~3.5 times lower than water intrusion. [μL/t or mL/t (where t = time)]
- The water intrusion value is referred to as the increase in compressed gas volume, because of water flow, normalized to atmospheric pressure. [NmL/t (where t = time)]
Working Principle
- Stabilization Phase: The pressure drop occurs during initial pressurization and stabilization due to:
- Cartridge deformation
- Removal of air within the membrane support layers
- Testing Phase: After stabilization of the system, the water volume remains to decrease, and this is caused by a combination of two separate mechanisms i.e., evaporation and intrusion into the pore.
Evaporation
Evaporation at the high-pressure downstream interface results in a reduced upstream water volume. This is the primary cause of water loss/flow leading to a pressure drop during the test phase.
Intrusion Into Pore Structure
Water gradually intrudes into the membrane over the course of the test, with the presence of larger pores in the filter being detected by an increased flow due to bulk water flow through pores.
If constant intrusion did occur through all the pores, it has been calculated that the membrane structure would completely wet out in approximately three hours. This does not happen as demonstrated by the fact that air can pass through the cartridge immediately after a water intrusion.

Critical factors to be considered during the development of the Automatic FIT cycle:
- The FIT cycle should be designed according to the issued set-points and water intrusion rates as recommended by the filter manufacturers. For example, the maximum allowable water intrusion rates for 0.2 μm standard Sartofluor® GA filters at a test pressure of 2.5 bar (i.e. 36 psi) and a temperature of 20°C as per 10″ element: 13 mL/10 min.
- Automated system shall be capable of recording initial and final pressure and temperature respectively.
- Capability of programming the calculation of water intrusion results by using the right formulae according to the allowed water inlet value in the filter manufacturers specification e.g. as below (zoom or open in new tab).
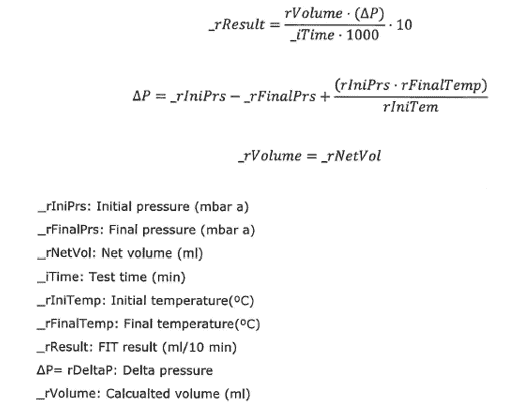
- Residual moisture content in the filter membrane should be less than 5% after the completion of FIT Cycle.
- Water Condition: Water quality is very significant factor to be considered to achieve the consistent results. It is recommended to use Purified water/WFI with a temperature range from 15℃ to 25℃; usage of potable water may result in contamination and uncertain results of your automated in situ filter integrity testing.
- Cartridge Condition: Cartridges should be free from contamination. Numerous factors can influence the filter cartridges contamination such as cleaning procedures, most commonly in the fermentation process there are more chances of contamination – [broth includes antifoams, and these can be deposited to the filter membrane in the form of aerosols]. Cartridge should be completely dried to get the best results for in situ filter integrity testing.
- Test Method: Housings and connections should be leak proof to get the accurate results from Automatic FIT. During testing of small filters systems maximum allowable water intrusion rates are extremely low, any leak in the housings, valves and connections may result in FIT failures.
Critical Parameters To Validate An Automated In Situ Filter Integrity Testing
- Test time (Typically 10 min)
- Allowed water intrusion (as per filter manufacturer specification)
- Initial stabilization and stabilization time.
- Draining time and drying time
- Initial and final temperature
- Initial and final pressure
- Net volumes
- Water intrusion value calculation formula (Program)
- Maximum pressurization loops and time
- Maximum filling time
- Residual moisture calculation (Typically calculated by initial and dried filter weight)
Potential Failures
- Pressurization failure: The activated value in the system sometimes not reaches the specified limits.
- Stabilization failure: Stabilization failure occurs when the temperature and pressure not stabilized in the initial phase.
Factors To Investigate In Case of Failure
- Filter (Cartridge) dryness
- Pore size correctness
- Any leaks in Housings, connections, and valves.
- Damages in gaskets or O rings
- Stabilization time
- Water quality and temperature
- Temperature and pressure.
Benefits of Automated In Situ Filter Integrity Testing
- The water intrusion test method is correlated to the liquid bacterial challenge which guarantees filter performance.
- In-situ FIT can be considered as operation friendly, no risk of manual removal and displacement of filters.
- There are no potential contaminants being introduced to the process as it uses high purity water and can therefore be integrity tested in-situ.
- Post testing drying cycle is shorter than equivalent drying cycle post solvent wetting.
The Downside
- The Automatic In-Situ FIT method is more time consuming than the pressure decay/diffusional flow test methods.
- WIT is suitable only in clean operating environments. The test is affected by contamination on the membrane surface, so should only be implemented on sterile gas cartridges which are adequately protected by both prefilters and steam filters.
Suitable Guidelines
- PDA Technical Report No. 26.
- FDA guide on Sterile Drug Products Produced by Aseptic Processing.
- American Society for Testing and Materials. “Standard test method for determining bacterial retention of membrane filters utilized for liquid filtration.” ASTM F-838-83. Philadelphia, PA 1993.
- European Medicines Agency. cGMP Note for Guidance on Manufacture of the Finished Dosage Form. London 1996 April Reissue: 5-6.
References
- ISPE technical publication 2017 – Risk Assessment for Thermal Influences on Filter & Container Closure Integrity Testing.
- Eu GMP Annex-1 and PDA TR 26.
- McBurnie L and Bardo B, Validation of sterile filtration. Pharmaceutical Technology, 2004 October.
- ICH Q7: Good Manufacturing Practice guideline for Active Pharmaceutical Ingredients
Conclusion
The filter integrity impacts the sterile process, as well as the compliance in the GMP environment. An automated in-situ FIT cycle must be configured in an appropriate way by:
- Qualifying the system within the validation process and
- Considering the aforementioned critical factors and CQAs.
This dramatically reduces efforts in the manual and displaced filter integrity testing procedure and should therefore be a preferred choice of standard integrity in-situ method for a filter integrity test.