Types of Reactors: Basic and Pharma Classification
Chemical reactions are integral to drug manufacturing. But the types of reactions differ. And accordingly, it requires different reactor types.
In chemical kinetics, reactions are classified through their rate and mechanisms with the aid of catalysts, which describe how the reaction is happening.
The catalyst is an additive in the chemical process that increases the rate of reaction without self-reacting.
Fundamentally, there are two types of reactions:
- Homogenous Catalysis (Reactant and Catalyst in the same phase)
- Heterogeneous Catalysis (Reactant and Catalyst in different phases)
Let’s understand the types of reactors based on such chemical applications.
Table of Content
Types of Reactors (Chemical Aspect)
We need extraordinary control to systematically convert reactants into products during such chemical reactions. Such controlling piece of an equipment is called reactor.
Therefore, selecting the type of reactor for a specific product depends on the expected outcome of the reaction mass.
Also, operating conditions like pressure, temperature, and mixing speeds become crucial to have efficient process runs.
Based on this, reactors are selected from the following different types.
- Batch (Batch and Semi-batch)
- Continuous (Continuous Stirred Tank Reactor)
- Tubular (Plug Flow Reactor)
- Catalytic (Fixed and Fluidized Bed)
Remember, these are the fundamental classifications of reactors and not the typical ones. Sub-types of these are briefed further (Image credits to Wikimedia).
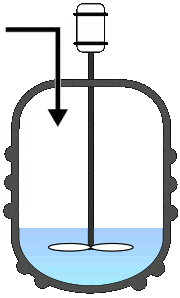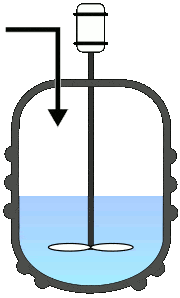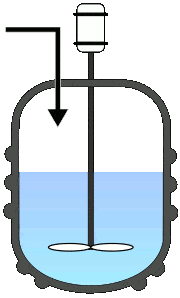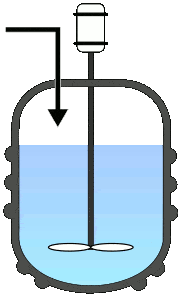
Batch Reactors
Once the reactants are added to the reactor, no third component is introduced, and also no component is taken out until the reaction completes. This will allow the process to run as a single batch until it produces the desired level of process conversion.
Semi-batch operations involve the progressive addition of one or more reactants during the reaction till it achieves the desired conversion.
Else, the reactants were added at once and progressively removed the converted product. Among all the fundamental reactors, this type is used frequently in pharmaceutical industries.
Here are a few highlights of the ideal batch reactor.
- Uniform composition throughout the reactor.
- Non-steady state mostly.
- They are more cost-effective in terms of instrumentation and construction than the continuous type of reactors.
- The size of the reactor depends on the volume of material that is to be charged instead of reaction time.
- For small capacity plants that handles the requirement of 4 to 5 tonnes/day, batch reactors are more economical than other types.
Though being the above highlights, it has some cons too.
- It requires tight temperature control due to the poor handling of highly heat-sensitive reactions.
- The quality of each batch may vary.
- Consumes significant resources.
A typical representation of batch reactors:
Continuous (Steady-State)
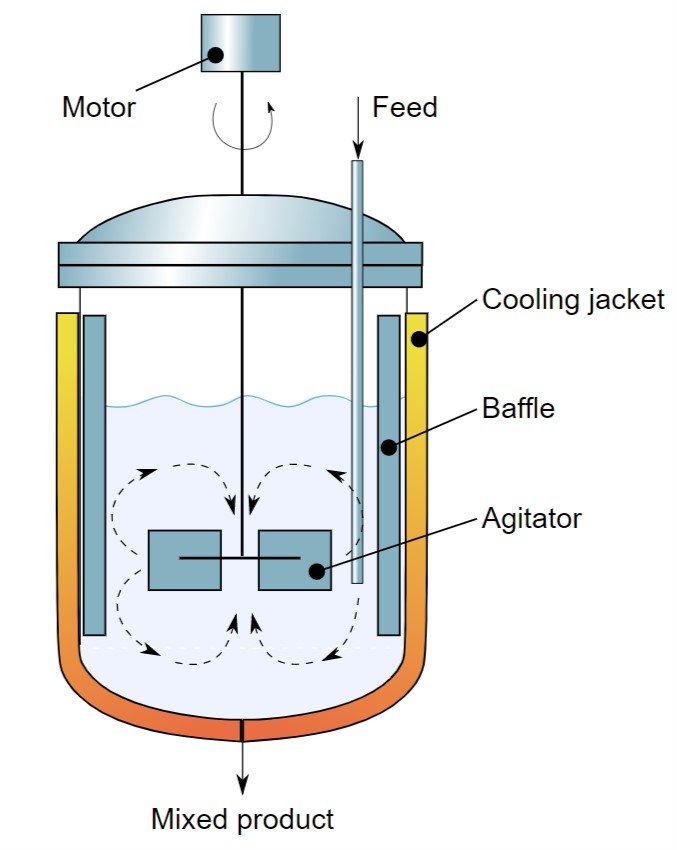
Unlike the batch reactor, a continuous stirred tank reactor carries out the reaction simultaneously, i.e. reactant addition and product removal simultaneously.
The level of the reaction mass is maintained constant such that the internal composition of the reaction mass is the same as discharged mass. Apart from other types of reactors, this type involves more common use in chemical process plants.
Here are few highlights of the continuous flow reactors.
- Being at steady-state, composition at any point does not change with time.
- Optimum quality parameters can be maintained for desired product conversions.
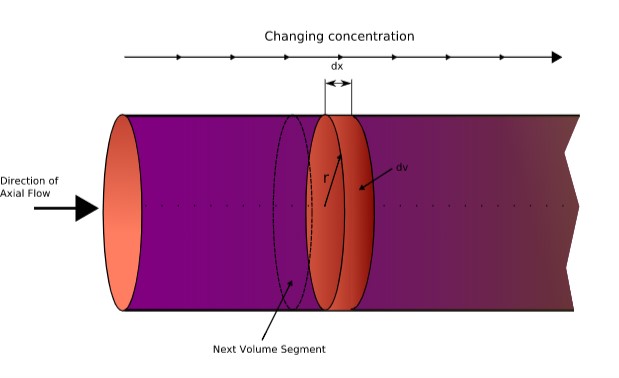
Tubular/Plug Flow Reactors
Tubular types of reactors such as Plug Flow Reactors (PFRs) have cylindrical tubes that mainly carry out the reaction.
Reactants enter at one end, react, harvest the product while traveling through the tube, and exit at the other end.
They are more efficient than CSTR for the same volume because of turbulent flow at the inlet. They generally have a flat velocity profile resulting in radial mixing.
The time the fluid particle spends in the plug flow is called ‘residence time’ and it is same for all the particles travelling through PFR.
Here are a few highlights of the ideal plug flow reactor.
- No axial mixing is permitted i.e., no chance of diffusion between the different particles traveling through the reactor.
- The composition of the reaction mass is unchanged with time. Rather, it changes with the length of the reactor.
- Requires less volume to yield the same conversion as that of continuous steady-state reactors for any positive order reaction.
- It does not have any moving parts, therefore, promoting highly pressurized and corrosive reactions.
Catalytic Reactors
The driving force for these types of reactors involves heat transfer, mass transfer, and mainly catalysts. Applications include Chemical Synthesis, Polymerization, Hydrogen Cracking, etc.
The common classification of these reactors is decided by the movement pattern of catalysts as given below:
- Fixed Bed
- Trickle Bed
- Fluidized Bed
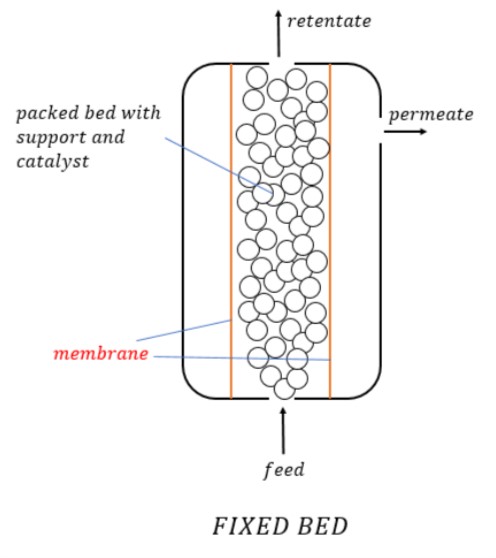
1. Fixed Bed Reactors
A fixed bed of catalyst is used to carry out heterogeneous catalyzed gas-phase reactions. They can be built on single or multiple tubes as per requirement.
The two types of reactors in the Fixed Bed category commonly include Packed Beds and Multi-tube reactors.
2. Trickle Bed Reactors
Used most commonly for hydrogenation reactions, trickle bed reactors have a solid phase as a catalyst for carrying out gas-liquid reactions.
It introduces liquid reactants from the top and flows downward contacting the solid catalyst bed while it introduces gas reactants from the cross current direction for better contact surface area.
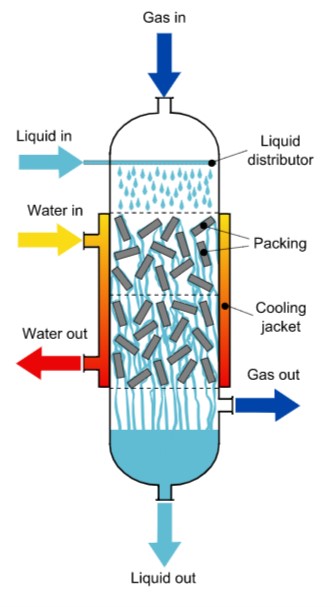
3. Fluidized Bed Reactors
A bed of solid particles is supported with a pull of gas or liquid flowing in an upward direction at a velocity such that the solid bed behaves as a fluid. This phenomenon is known as Fluidized Bed and the reactor used for this is called Fluidized Bed Reactor.
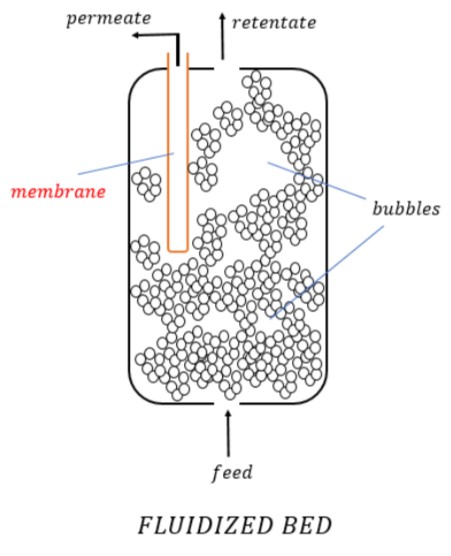
This was an overall picture of the fundamental classification of reactors and not an exhaustive one.
Below are the types of reactors based on the material of construction called out commonly in pharmaceutical-related industries.
Pharmaceutical Classification
In life-science applications, reactor types are based on the material of construction and process of interest specifically;
- Stainless Steel Reactors (SSR)
- Glass Lined Reactors (GLR)
The application and selection of these types of reactors depend on the scope of the process. For any chemical process, there are three natures:
| Nature | pH | Appropriate Type |
|---|---|---|
| Acidic | <7 | Glass Lined Reactors (GLR) |
| Alkaline | >7 | Stainless Steel Reactors (SSR) |
| Neutral | =7 | Stainless Steel Reactors (SSR) |
Stainless Steel Reactors (SSR)
Different medicines have different safety requirements and level of stringency. For example, Bulk drugs have less stringent requirements than biosimilars.
Accordingly, there are two common types of reactors based on the material alloy configuration, i.e., SS304 and SS316.
You can find more information in the ASTM guidelines for this, still let’s quickly see in short.
SS304 contains approx. 18% chromium and 8% nickel while 316 contains 16% chromium, 10% nickel and 2% molybdenum. This 2% molybdenum helps to survive in a corrosion-promoting environment.
Hence, SS316 is more advanced than SS304. However, visible differentiation is not possible between these two and requires a material test report from the vendor or tester to conclude it as SS316L.
Also, if carbon is a problem, preference is given to SS304L or SS316L. In this, L stands for low carbon content. Regular 304 or 316 contains ~0.09% carbon, while the ‘L’ version has around 0.03% carbon. Carbon also contributes to corrosion during very high-temperature operations.
The bottom line is that Stainless Steel Reactors are more suitable for neutral or alkaline process operations. Though sometimes, weak acids like acetic acid can satisfactorily be processed in SS316L with a pH value as low as 3 due to its less corrosion impact than other strong acids.
Glass Lined Reactors (GLR)
When dealing with strong acids like Nitric, Sulphuric, Hydrochloric, or Phosphoric acids, stainless steel reactors are non-sustainable and hence corrode/dis-integrate over time.
To avoid that, reactors with glass lining as thick as 0.2mm are used because glass easily sustains acids. Glass offers complete protection against strong acids preventing wear and tear in the long run.
Glass Lined Reactors are not suitable for alkaline operations because borosilicate commonly used in glass easily reacts with high pH solutions and degrade.
Alkalis are more corrosive than acids, especially at elevated temperatures. This may cause cracks in the glass, which is undesired. However, GLRs are more sustainable to a pH value of 8.5 i.e., until you use weak bases.
Stainless Steel Reactors possess a better heat transfer effect because of enhanced thermal conductivity. Apart from this benefit, GLRs have restricted operation at high pressures.
Though being easy to clean, lightweight, cost-effective, and corrosion-resistant, GLRs have a delicate physique that requires careful handling.
GLRs are seen in various colors according to their suitable purposes such as Blue, Light Blue, Green, and White. Characteristics of each color in short.
- Blue – Higher thermal conductivity, a wide range of pH acceptance
- Light Blue – Highly resistant to alkalis, easy for visual observation
- Green – Suitable to operate under high temperatures up to 300ºC
- White – Easy visual observations in colorful chemical reactions like photochemicals.

Above all, these types of reactors (SSR and GLR) require different specialized instruments according to their MOC like agitators, baffles, temperature probes, pH probes, valves, manholes, etc. as per their CIP and SIP requirements.
Hope you got an overall idea of how complex but systematic is the classification of reactors as per different applications they are used for.
Did you find something difficult to digest from this post? Or you loved it? Either way, let me know in the comments.
Here is the FAQ section:
SSR and GLR: FAQs
What is SSR reactor?
Process vessel or reactor having material of construction as Stainless Steel, either SS316L or SS304 for product contact parts. SSRs are more suitable for neutral or alkaline process operations. Though sometimes, weak acids like acetic acid can satisfactorily be processed in SS316L with a pH value as low as 3 due to less corrosion impact compared to other strong acids.
What is GLR reactor?
Reactors are made up of glass that has borosilicate as a major component to handle acidic reactions known as Glass Lined Reactors. The u0022Linedu0022 word shows the application of glass lining inside the stainless steel reactor to handle strong acids. These are not suitable for handling highly corrosive alkalies.
Why is the glass lined reactor Blue?
The composition of cobalt in glass causes it to produce a blue color. Blue color exhibits higher thermal conductivity and a wide range of pH acceptance. To read more, please read the above article for the purpose or application of color differentiation within the Glass Lined Reactors.





Thank you sir … upload HFF & UFF systems process & CIP,ESIP, etc,,,
Thanks for reading and sharing your interest!
Yes, HFF (Hollow Fiber Filtration) and UFF (Ultrafiltration) systems deserve their own deep dive, especially around:
• Process flow
• CIP (Clean-in-Place)
• ESIP (Enhanced Steam-in-Place)
• Integration into upstream/downstream operations
Very good info. Which reactor type is suitable for high pressure/high temperature operations (10 bars, 150C) with acetic acid being one reactant and final compound has metal leaching nature?
Great question.
For high-pressure, high-temperature reactions involving corrosive agents like acetic acid and potential metal leaching:
Go for a Hastelloy-clad high-pressure reactor.
• Handles 10+ bar and 150°C with ease
• Excellent corrosion resistance vs acetic acid
• Reduces risk of contamination from metal leaching
• Long lifecycle, despite harsh conditions
Glass-lined reactors won’t cut it here. Stainless steel may leach metals.
Hastelloy is your best bet.
Glad to know this information
Thanks
Useful information thank you sir
Thanks
Thank you for the valid information. It is highly helpful for me to undergo interview.
Glad it helped you! You’ve got this
As we consider DIN Standards for GLRs, do we have standards for SSRs? If yes, what are they?
Great question. While DIN standards are well-established for Glass-Lined Reactors (GLRs), Stainless Steel Reactors (SSRs) don’t follow a single unified DIN standard. Instead, they are typically designed per:
• ASME Section VIII, Div. 1 (for pressure vessels)
• GMP guidelines (for pharma-specific cleanliness and construction)
• PED (EU Pressure Equipment Directive) if exported to Europe
• IS 2825 (Indian standard for pressure vessels)
The choice depends on process, region, and regulatory expectations. Always align with intended use and compliance scope.
Thanks for this amazing and useful information. Great job.
Thanks
I got full information about the reactors. This topic is so good to understand things easily. Thanks, and great work.
Thank you so much for the kind words. Glad the article made complex concepts easy to grasp. That’s the goal: Simplify the science without watering it down. Stay tuned for more deep dives.